Our story begins at the world-famous Cavendish Laboratory at Britain’s Cambridge University. A twenty-four-year-old scientist named Joseph John “J. J.” Thomson (1856—1940) arrived there in 1881, and he found that the atmosphere there was “electric” in more ways than one. The laboratory’s founder, James Clerk Maxwell (1831–1879), was famous for formulating a set of four equations that described the relationship between electricity, magnetism, and light waves. Thomson hoped to follow in his footsteps.
Other major discoveries of that time came from chemistry. By studying the reactions between different substances, chemists had concluded that matter was composed of atoms, and that atoms combined in particular ways to form molecules. They thought those atoms were indivisible. They also had discovered that electricity was related to chemical reactions. But where the electrical nature of matter came from remained a mystery.
Cathode Ray Experiments
Young Thomson wanted to use his mathematical gifts to make discoveries about the electrical nature of matter just as Maxwell had done for the electromagnetic nature of light. But his boss, John William Strutt (1842–1919), more commonly called Lord Rayleigh, who became Cavendish Professor in 1879 after Maxwell’s death, had other plans. Rayleigh believed that in physics, mathematical skills should not stand alone. If Thomson intended to work at the Cavendish, he would have to do more than calculation. He would have to work in the laboratory!
Unfortunately, Thomson was not very skilled with scientific apparatus. His contributions to experiments were more of the mind than of the hand. “J. J. was very awkward with his fingers, and I found it very necessary not to encourage him to handle the instruments!” said H. F. Newall (1857–1944), Thomson’s assistant in his early years at the lab. “But he was very helpful in talking over the ways in which he thought things ought to go.”
Thomson’s most important investigations were into the well-known but poorly understood phenomenon of cathode rays. The cathode ray tubes Thomson used were primitive versions of those used in old-fashioned television picture tubes. As early as the 1830s, scientists were experimenting with the electrical behavior of gases in glass tubes with two electrodes inserted at opposite ends. They needed low pressures for their experiments, so they pumped hard on the tubes to draw out most of the gas before sealing them. The invention in 1855 of a better vacuum pump made it possible to remove nearly all the gas, and things began to get very interesting when electricity was applied. The remaining gas would glow, especially near the negative electrode, or cathode.

Joseph John (J. J.) Thomson’s work with cathode rays led him to discover the electron in 1897.
Particles or Waves?

Answering a great question in a famous 1801 experiment, Thomas Young produced an interference pattern of light and dark bands that demonstrated the wave nature of light.
In the history of science, one of the great questions has been about the nature of light. It first came to prominence in the seventeenth century, when two great thinkers came to opposite conclusions. England’s Sir Isaac Newton (1648–1727) was convinced that light was a stream of particles, while Christiaan Huygens (1629–1695) of the Netherlands was just as certain it was made of waves.
Without evidence to support one view or the other, scientists argued about it for more than one hundred years. Finally in 1801, Thomas Young (1773–1829) settled the question with an experiment. He passed light through two closely spaced pinholes in front of a white screen. Instead of seeing two bright spots as he would expect if the light was a stream of particles, Young observed a pattern of dark and light bands called an interference pattern that would be expected if two waves met. At places on the screen where two wave crests met, it was bright. At other places, wave crests met valleys and cancelled out, producing darkness.
It seemed that the argument was settled in favor of Huygens—though you will read later that it wasn’t as settled as scientists thought. At the Cavendish, J. J. Thomson would revisit the wave-or-particle question for a phenomenon called cathode rays. And his findings would turn out to be just as dramatic and important as Young’s and Maxwell’s were for light.

J. J. Thomson’s Cathode Ray Tube. The development of better vacuum pumps enabled J. J. Thomson to use this apparatus to determine that cathode rays were streams of negatively charged particles, which today we know are electrons.
When Thomson began studying cathode rays, scientists knew that the cathode was shooting out tiny, negatively charged particles. But they were divided about whether the particles themselves were the cathode rays, or whether the glow resulted from waves that the particles produced. Thomson hoped to settle the question with an experiment.
As you read in “Particles or Waves,” the question of whether cathode rays were waves or particles echoed a similar question about light. A famous 1801 experiment performed by Thomas Young seemed to settle the question in favor of light waves. And that view became even stronger in 1861–62 when Maxwell published his four famous equations. Those equations predicted waves of electromagnetic energy that travel through space at the speed of light.
For cathode rays, however, there were no equations like Maxwell’s, and so far, experimental results had been mixed. So Thomson kept an open mind and began by repeating what others had done.
He applied magnetic fields to the tubes, and the rays curved in the direction that the magnetic field would cause negatively charged particles to curve. But when he passed the beam between a pair of oppositely charged electrified plates, the cathode rays went straight through, producing a glowing spot on the center of the glass. If cathode rays were streams of negative particles, the glowing spot on the glass should have been offset in the direction of the positively charged plate—but it wasn’t.
Maxwell’s Equations and Electromagnetic Waves
Although you need to know calculus to understand the mathematics of Maxwell’s equations, it is fairly easy to understand the phenomena that they describe. One phenomenon is the force between two electric charges. In a famous experiment in 1785, Charles-Augustin de Coulomb (1736–1806) measured how that force depends on the size of the two charges and their separation. He discovered that the force behaved in the same way as the gravity between two bodies, except that it can be either attractive (if the charges are of opposite sign) or repulsive (if the charges have the same sign).
That relationship is a formula known as Coulomb’s Law and contains a natural constant that is needed to relate the units used to measure force (such as pounds or newtons) to the charges (measured in coulombs) and the separation (measured in feet, meters, or centimeters). Scientists call that constant ε0 (epsilon sub-zero, where the zero means the charges have no material between them). A similar equation relates the force between two magnetic poles and contains a constant μ0 (mu sub-zero).
The other two phenomena relate electricity to magnetism. The best-known experimenter in that area was Michael Faraday (1791–1867) in the 1820s and early 1830s. Faraday discovered that electric coils can produce a magnetic field and that a changing magnetic field can generate an electric current. His principles are still used today in electric generators and motors.
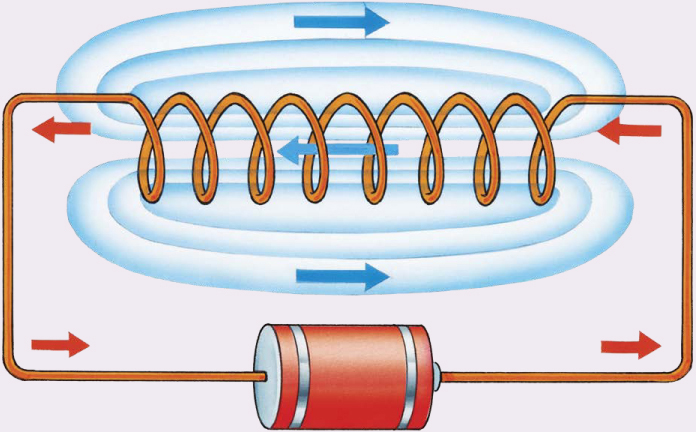
A simple electromagnet. A battery connected to a coil of wire produces an electric current (shown by the red arrows) and causes the coil to act like a bar magnet. The blue lines and arrows show the direction that a compass needle would point when placed in or near the coil.
When the equations are combined, they predict the possibility of electromagnetic waves, whose speed can be calculated from ε0 and μ0. Astonishingly, the calculated speed of those waves turns out to match the measured speed of light.
Thomson then devised three new experiments. In the first, he put an electrometer, a device that measures electrical charge, into the tube. When the glow struck the electrometer, the device indicated a large negative charge. When the glow just missed, the electrometer measured very little charge. Thus cathode rays were either a stream of negative charges, or they carried such a stream with them.
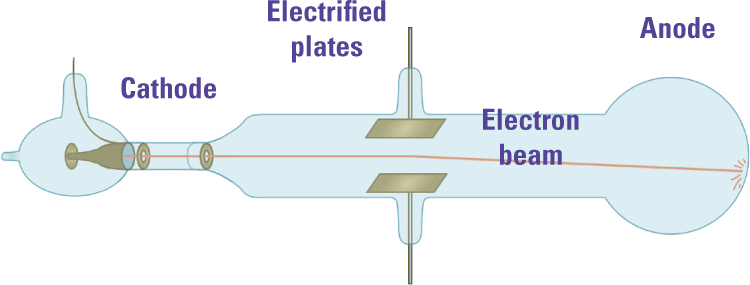
Thomson’s Second Experiment. Using an improved vacuum pump, J. J. Thomson repeated earlier experiments that had not produced clear results. With almost no gas left in the tube, the cathode rays curved toward the positively charged plate (the lower plate in this diagram), demonstrating that they were negatively charged particles.
Thomson’s second experiment clarified the puzzling results with the electrified plates. He reasoned that an energetic beam of negative particles would electrify the gas it passed through, and the charged gas atoms would drift toward the oppositely charged plate, neutralizing the electric field within the tube. Thomson believed that if he could do a better job of removing the gas from the tube, there would be too few molecules to neutralize the electric field. He got the best available vacuum pump and repeated the experiment. Sure enough, the cathode rays now deflected toward the positive plate. Thomson was now confident that they were negatively charged “corpuscles,” that is, particles shot from the cathode.
The second experiment also enabled Thomson to measure the speed of those tiny particles. He added a magnet to the apparatus. The magnetic force on the corpuscles depended on their speed, but the electrical force did not. Thomson arranged the fields so that the magnetic and electrical forces were in opposite directions, and then adjusted the fields until the particles went straight, indicating that the forces were equal. Knowing that, he was able to compute the speed from the strength of the two fields.
Thomson was then was ready for a final experiment that would allow him to compute the corpuscles’ electric charge compared to their mass. He allowed just enough gas back into a tube so that the cathode rays’ glowing path could be seen and measured precisely. He applied a magnetic field and measured the curvature of the path. Knowing the speed, the curvature, and the formula for magnetic force, he was able to use his results to measure the charge-to-mass ratio of the corpuscles.
He discovered that the charge to mass ratio was about one thousand times as large for these corpuscles as for an electrically charged hydrogen atom, the lightest particle known at that time. For that to be true, the corpuscles either had to carry a lot of charge or have very little mass. Other scientists’ measurements indicated that there was a basic minimum unit of electric charge for both positive and negative electricity, so Thomson assumed that each negative corpuscle and each positive hydrogen atom carried the same amount of electrical charge. That meant that the corpuscle had only a thousandth of the mass of the tiny hydrogen atom. Today, we know more precisely that this particle’s mass is only 1/1,837.15 of the hydrogen atom.
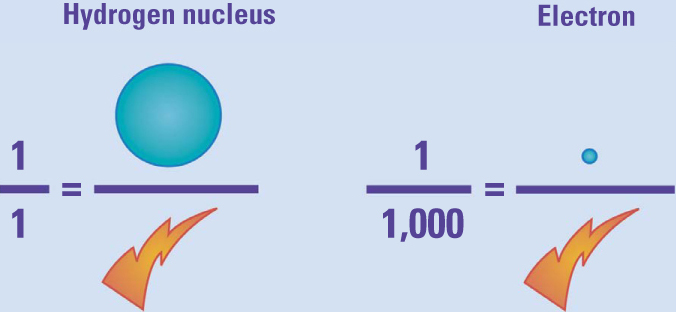
Thomson’s third experiment measured the charge-to-mass ratio of the “corpuscles” in cathode rays. The corpuscles carried the same amount of charge in less than a thousandth of the mass of a positively charged hydrogen atom. More precise measurements have since revealed a ratio of approximately 1,837-to-1.
The result was astounding. Cathode rays were beams of particles, as Thomson had suspected all along, but their mass was far smaller than he could have imagined. In his 1906 Nobel Prize lecture, Thomson still called the particles “corpuscles.” But he realized their importance went far beyond cathode rays, stating, “The corpuscle appears to form a part of all kinds of matter under the most diverse conditions; it seems natural therefore to regard it as one of the bricks of which atoms are built up.”
In other words, Thomson was stating that his corpuscles were subatomic particles. It was not long before people began calling them electrons. The door to the subatomic world had opened.