3 HOW
Electrons Explain Chemistry
I flight, long to be thought of as a wave phenomenon, could sometimes behave like a stream of particles, could particles such as electrons sometimes behave like waves? In 1924, a French physics student named Louis-Victor de Broglie (18921987) explored that question in his doctoral dissertation. His answer was a clear yes. He devised a formula that used Planck’s constant to relate an electron’s wavelength to its speed, and he discovered that the circumference of the allowed orbits in Bohr’s theory was a whole number of electron wavelengths.
Now the distinction between particles and waves had blurred completely, and other physicists struggled to find new ways to understand the laws of motion within the atom. Among them was Erwin Schrödinger (1887–1961), who developed an equation that described a particle’s position by a mathematical formula called a wave function. Schrödinger’s equation launched a new field of physics called quantum mechanics. Strangely, a particle was no longer considered to be in an exact place. Rather, the particle’s wave function gave the probability of finding it in many different places.
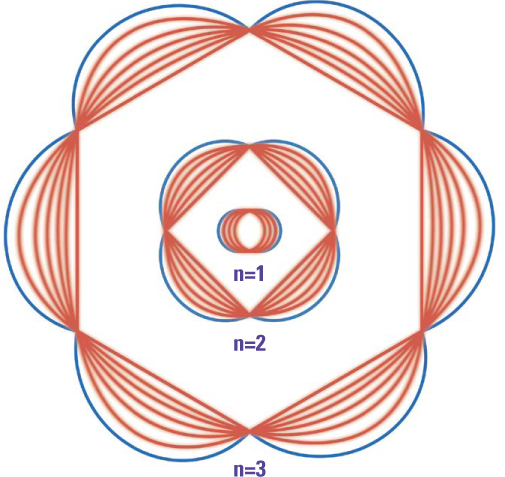
In his 1924 doctoral dissertation, Louis de Broglie launched a new approach to quantum mechanics by extending Planck’s idea that light had both wavelike and particle-like properties to electrons. In Bohr’s model of the atom, electrons could orbit in certain allowed energy levels. De Broglie replaced that with electrons as waves wrapped around a nucleus. The circumference of the allowed orbits was a whole number of electron wavelengths.
To understand what that means, imagine an object bouncing back and forth so fast on a very tight spring that all you can see is a blur. Near the ends of its bounces, the object moves more slowly and the blurriness decreases. So where is the object? It could be anywhere along the path, but it is more likely to be near one of the less blurry ends than in the blurry middle. In quantum mechanics, the blur is the object.
When Schrödinger applied his equation to the hydrogen atom, it produced a series of different electron wave functions, each concentrated at a certain distance from the nucleus and each had a certain energy. The distances, orbital shapes, and energies were the same ones that Bohr, Sommerfeld, and de Broglie had calculated.
How Large Is an Atom?
Quantum mechanics provides the answer to many puzzling aspects of the subatomic world. For example, it helps explain how, if the nucleus is so small and electrons have so little mass, an atom can be so much larger than the nucleus. The answer is that you can’t simply think of an electron as a tiny particle following an orbit; you have to consider its wavelike properties as suggested by Louis de Broglie. Instead of being in a particular spot on the orbit, its wave function means that it is everywhere in that orbit all the time.
As you will read later in this chapter, each electron in an atom is in a different orbit. The size of the atom is therefore the size of the largest orbit

Louis de Broglie in 1929, the year he won the Nobel Prize that has an electron.

The Modern Periodic Table includes many elements that were undiscovered when Mendeleyev first proposed it. It also reverses the roles of rows and columns from Mendeleyev’s original arrangement.

Building on an idea of Louis de Broglie that electrons and other particles have wavelike properties, Erwin Schrödinger devised an equation that represented particles as “wave functions” that change in space and time.
Quantum Mechanics and the Periodic Table
As physicists looked more deeply, they discovered that quantum mechanics could also explain one of chemistry’s great mysteries. What underlies the regularity of the periodic table of the elements discovered by the Russian chemist Dmitry Ivanovich Mendeleyev (1834–1907) in 1869?
Mendeleyev had arranged the known elements by increasing atomic weight into columns and then rows. Modern periodic tables order the elements by rows and then columns. All the elements in one of Mendeleyev’s rows formed similar compounds. In today’s table, the properties are similar down a column.

Quantum Jumps. As quantum mechanics evolved, energy levels and electron wave functions replaced Rutherford’s idea of orbiting electrons. Bohr’s model of the atom, in which electrons make quantum jumps between energy levels and emit quanta of light, explained the observed line spectra of various elements. It also removed Rutherford’s concern that orbiting electrons would radiate energy and spiral into the nucleus.
For example, sodium is above potassium in column I, and both form chloride molecules with one atom of chlorine. Their neighboring atoms in column II—magnesium and calcium—form chloride molecules containing two chlorine atoms. After the electron was discovered, it soon became apparent that the different number of electrons in atoms determined their placement in Mendeleyev’s columns. It took quantum mechanics to reveal the explanation.
In quantum theory, each electron orbit in an atom can be labeled by a set of quantum numbers. The principal quantum number, called n, is equal to the number of electron wavelengths that fit into that orbit. The electron’s “orbital” quantum number, denoted by the letter l, describes how elliptical the orbit can be. The value of l must always be less than the principal quantum number n. Thus when n is one, l must be zero (a circular orbit). When n equals two, l may be zero or one.
Filling Up the Periodic Table
Mendeleyev’s original periodic table was different from the modern periodic table in significant ways. In Mendeleyev’s time, chemists were able to measure the atomic weight of elements, but they were not aware of electrons or the nucleus. Modern chemists know about the atomic number, which is the positive electric charge of an element’s nucleus. That number corresponds to the number of electrons when the atom is in its electrically neutral state.
Mendeleyev first conceived the periodic table of the elements in 1859.
Mendeleyev ordered the elements down columns and then across rows by increasing atomic weight. The modern periodic table orders the elements across rows and then down columns by increasing atomic number. Usually an element with a larger atomic number also has a larger atomic weight, but there are a few exceptions. So besides the reversed role of rows and columns, the order of elements is slightly different today than in Mendeleyev’s time.
The most important difference between the tables is the gaps in Mendeleyev’s table where elements had not yet been discovered. In order to group elements with similar properties across his rows, Mendeleyev left blank spaces. He predicted that elements would be found to fill in those spaces, and he described their properties. When they were later discovered, it was a great triumph for his idea.
Mendeleyev left out an entire group of elements, which are known today as the noble gases. Those elements—helium, neon, argon, krypton, xenon, and radon—have no valence electrons and thus do not react chemically without a lot of added energy. Sir William Ramsay (1852–1916) and Lord Rayleigh—whose insistence that J. J. Thomson do experimental work led to Thomson’s discovery of the electron—were the first to discover these gases.
A third quantum number, called m for magnetic, envisions the nucleus as a sphere with a north-south axis. An electron’s orbital plane can go around the equator (m=0), or it can be tilted a different amount, corresponding to the value of m. The value of m is less than or equal to the value of l. And m can have either a positive or negative sign, depending on whether its orbital direction is clockwise or counterclockwise.
Finally, just as Earth spins on its axis once per day while orbiting the Sun once a year, an electron also has a spin quantum number, denoted by s, which can only be in one of two states, often called spin up (s=+1/2) and spin down (s=-1/2). No two electrons in the same atom can have the same “quantum state,” or the same set of values for n, l, m, and s. Physicists discovered that electrons would fill the quantum states in a predictable order. Some electron arrangements were especially favorable. Those are the noble gases (see Filling Up the Periodic Table on pages 40-41), and larger atoms would build up from those as a core.

Molecules are formed when two or more atoms bond together, usually in a way that fills an electronic shell or subshell. One way to do that is through sharing electrons in a covalent bond. For example, two oxygen atoms bond covalently to form an oxygen molecule. Each atom’s outer shell has six electrons, two short of the eight needed to complete that shell. Two oxygen atoms bond when each shares two of its outer electrons with the other, completing the outer shells of both.
Atoms in the same column of the periodic table have the same pattern of electrons in their outer orbits around different cores. Those outside electrons, called valence electrons, are responsible for an atom’s chemical behavior. Quantum mechanics explained why the periodic table is periodic!
Chemical Bonds
Once that explanation was known, physicists and chemists began speaking of electrons filling “shells,” which were sets of all the quantum states having the same value of n, or “subshells,” which were smaller parts of those shells having certain values of l and m. In any column of the periodic table, the core electrons were always in completely filled inner shells and subshells, and the valence electrons were in partially filled outer subshells.
The fewer valence electrons there are, the easier it is to remove them. The elements in column I of the periodic table, sodium and potassium, for example, have only one valence electron, which they give up easily. On the opposite side of the periodic table, the valence electrons are close to filling a subshell, and thus have greater attraction not only for each other but also for other electrons that may be nearby. Fluorine and chlorine in column VII, for example, need only one more electron to complete a subshell.
When a chlorine atom and a sodium atom get close to each other, the chlorine snatches the sodium’s valence electron, leaving both as electrically charged “ions” with filled subshells. The positively charged sodium ion and the negatively charged chloride ion experience a strong electrical attraction—an ionic bond—that results in a sodium chloride molecule.
Other kinds of atoms, especially those in the center columns of the periodic table, may join together by sharing rather than exchanging electrons. That is called covalent bonding. For example, a nitrogen atom has three valence electrons in a subshell that can hold six. When two nitrogen atoms share all their valence electrons in a covalent triple bond, it completes the subshells of both. The result is a two-atom nitrogen molecule. Oxygen atoms too, with four of six possible electrons in their outer subshells, share two valence electrons each in a covalent double bond to form two-atom oxygen molecules.
Metals get their special properties from a different type of chemical bonding. Each atom shares all of its outer electrons with every other atom, so the metallic bonds are not between pairs of atoms but rather between each atom and the whole. As a result, those shared electrons are bound only weakly to their nearest atom and flow easily to the next one, explaining why metals carry electricity so well. The shared electrons are thus often called “conduction electrons.”
Metallic bonding is also involved in both the photoelectric effect and cathode rays. If a strip of metal is placed in a beam of ultraviolet light, each photon carries enough energy to free a conduction electron. Likewise, the electric field in a vacuum tube is strong enough to pull some conduction electrons from a metal cathode.
In a way, all of chemistry is the study of electrons—from the periodic table to understanding how atoms bond with each other through sharing or exchange of electrons. That is also true of much of modern technology. Understanding how to control electrons has led to an array of electronic devices that are changing the way people live, work, and communicate no matter where in the world they live.