CHAPTER 5
The African INTERHEART study
Krisela Steyn1 and Karen Sliwa2
1 Medical Research Council, Tygerberg, South Africa
2 University of Cape Town, South Africa; University of the Witwatersrand, Johannesburg, South Africa
5.0 Introduction
With the African continent so large and diverse, from industrialized cities where Westernized lifestyles have become the norm to more remote rural regions where traditional lifestyles still predominate, there are inherent disadvantages on focusing on specific communities and regions. As already suggested by potentially discordant data from South Africa and Nigeria, relative to each other and to historical reports, the phenomenon of epidemiological transition towards noncommunicable forms of heart disease is far from uniform. Indeed, it is subject to the vagaries of geography, culture, and economic development across the continent. Large-scale registries or observational studies that collect less detailed but uniform data across a range of populations play an important role in understanding both the common and unique factors driving any particular disease state from a national to global perspective. It was on this premise that the Global INTERHEART Study (an observational case-control study) examined the pattern of risk in cases of AMI across 52 countries (including representative data from Africa) [64]. Predictably, perhaps, the study demonstrated that regardless of the population, the same risk factors (see Figure 5.1) feature heavily in this common manifestation of CAD.
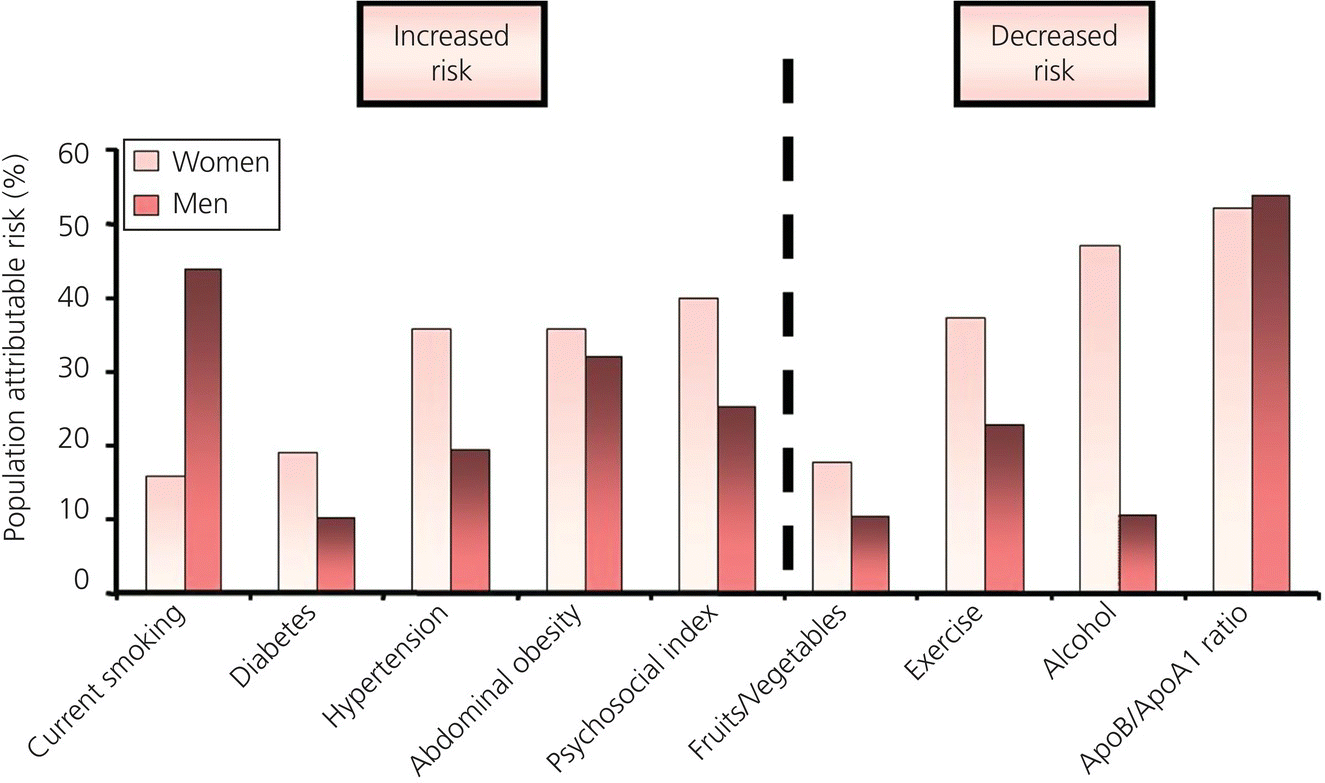
Figure 5.1 Pattern of risk factors for AMI according to sex.
5.1 The African INTERHEART study
Steyn K, Sliwa K, Hawken S, Commerford P, Onen C, Damasceno A, Ounpuu S, Yusuf S. Risk factors associated with myocardial infarction in Africa: the INTERHEART Africa Study. Circulation 2005; 112(23):3554–61. [64]
5.1.1 Study aims
Following the release of the major findings from the global INTERHEART study, a more detailed analysis of the study data from Africa was published. The African INTERHEART study investigated the pattern of AMI and risk within the African population. Specifically, it examined the differences in the risk profiles of three distinct ethnic/cultural African groups to identify any key differences in epidemiological transition between groups. The three target groups (as labeled) were those of African ancestry, colored Africans, and European/other Africans.
5.1.2 Study methods
5.1.2.1 Patient enrollment and data collection
From 1999 to 2003, the African INTERHEART study investigators collected data on 578 de novo cases of AMI and 785 age- and sex-matched controls from nine sub-Saharan African countries (Benin, Botswana, Cameroon, Kenya, Mozambique, Nigeria, Seychelles, South Africa, and Zimbabwe). Cases of AMI were derived from systematic screening of patients in the medical wards or coronary care units of participating centers (with relevant data collected within 24 hours). Case controls were derived from visitors to the participating centers. Trained staff collected profiling data from the participating sites in a standardized manner: sociodemographic profile, family cardiovascular history, lifestyle behaviors (including smoking habits and physical activity), and clinical history (including a history of hypertension and/or type 2 diabetes) were included. Active measurements of anthropometric profile, mental health status, and lipid profile (available for 76% of the patients) were also obtained.
5.1.3 Study findings
5.1.3.1 Cohort profile
Overall, 81.2% of the patients were from South Africa (this represents an important caveat when interpreting study data), and the overall ethnic groups comprised 46.9% colored Africans, 36.4% of African ancestry, and 17% European/other Africans (see Figure 5.2). Nearly 66% were men. Once again, this profile provides an important caveat in any interpretation of study findings.
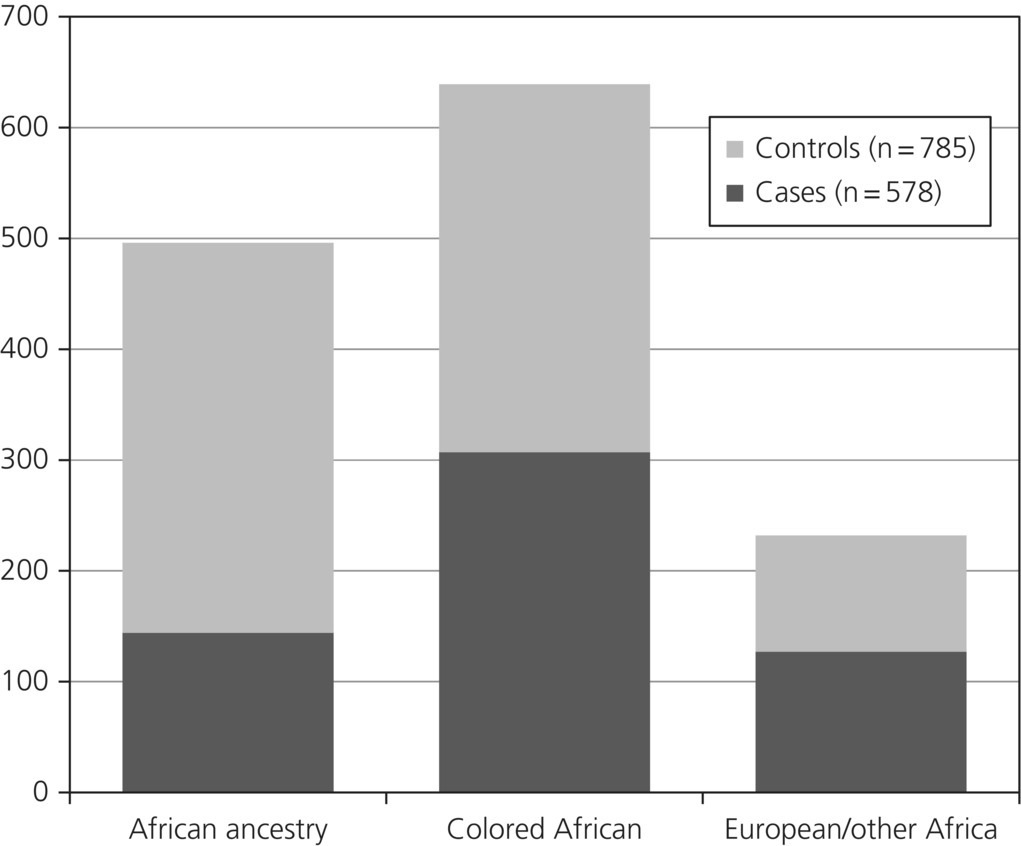
Figure 5.2 Cases and controls by ethnicity in the African INTERHEART Study.
5.1.3.2 Overall pattern of risk associated with a de novo AMI
Consistent with the overall pattern and natural history of AMI [9,65,66], in both the global INTERHEART study and the African INTERHEART study, there were more men than women cases (p < 0.0001), with men presenting younger than women (mean age of 53 ± 12 years versus 56 ± 11 years; p = 0.0014) (see Table 5.1). Across the three ethnic groups, the age of a de novo AMI was similar (mean age of 54 years), and overall, those of African ancestry were around 4 years younger than their international counterparts. The major risk factors linked to AMI and other forms of CVD around the globe were just as predominant in those of African ancestry, with a history of hypertension and diabetes representing the strongest risk factors in this regard. Consistent with the findings presented in Chapter 4 suggesting a greater role for hypertensive-related diseases, compared to case controls, hypertension was significantly more likely to occur in African ancestry cases than overall (OR of concurrent hypertension 3.4 versus 2.5; p < 0.01). Along with hypertension, abdominal obesity and apolipoprotein B (apoB)/apolipoprotein A-1 (apoA1) ratio were also more likely to present in AMI de novo patients of African ancestry (compared to controls). Overall, however, current and former cigarette smoking and stress were found to be the strongest risk factors in the African INTERHEART patients.
Table 5.1 Patients’ risk factor profiles according to the African INTERHEART Study and the Global INTERHEART Study.
| African INTERHEART Study | Global INTERHEART Study | |||
| Controls (n = 14,637) | Cases (n = 12,461) | Controls (n = 785) | Cases (n = 578) | |
| Men | 10,846 (74.1%) | 9,458 (75.9%) | 507 (64.6%) | 385 (66.6%) |
| Mean Age | 52.21±11.53 | 54.3±11.29 | 56.9±12.2 | 58.1±12.2 |
| Hypertension | 3,206 (21.9%) | 4,860 (39%) | 148 (18.8%) | 244 (42.3%) |
| Diabetes | 1,098 (7.5%) | 2,305 (18.5%) | 60 (7.6%) | 136 (23.6%) |
| Current cigarette smoking | 3,923 (26.8%) | 5,632 (45.2%) | 299 (38.1%) | 302 (52.3%) |
| Former smoker | 7,040 (48.1%) | 65.2 (65.2%) | 442 (56.3%) | 418 (72.3%) |
| Exercise | 2,825 (19.3%) | 1,782 (14.3%) | 133 (16.9%) | 87 (15.1%) |
| Alcohol intake | 3,586 (24.5%) | 2,991 (24%) | 210 (26.8%) | 128 (22.2%) |
| Daily fruits and vegetables intake | 6,206 (42.4%) | 4,461 (35.8%) | 309 (39.4%) | 216 (37.4%) |
| Depression | 2,561 (17.5%) | 2,991 (24%) | 175 (22.3%) | 184 (31.8%) |
| Stress | 629 (4.3%) | 959 (7.7%) | 35 (4.5%) | 55 (9.6%) |
5.1.3.3 Ethnic differences
Some important differences in the risk profiles of cases and controls were evident according to ethnicity. Consistent with community surveillance and the Heart of Soweto registry data, the lipid profile of African ancestry controls was more benign and less likely to lead to atherogenesis than those of the other ethnic groups. This included lower levels of low-density lipoprotein (LDL) cholesterol, total cholesterol, apoB/apoA1 ratio, and apoB (adjusted for differences in smoking status, sex, and age). In the African ancestry group, relative to those with less than 8 years of schooling, those with tertiary education had an elevated risk for AMI. The direction of this observed association between education level and de novo AMI was reversed in the European/other African group. In the latter group, less education was associated with an increased risk of presenting with an AMI. This same pattern was observed when the relationship between AMI and income was examined. Relative to those with the lowest income, those of African ancestry with the highest income were more likely to present with a de novo AMI (OR 2.75; 95% CI 1.53–4.94). Alternatively, in the European/other African group, higher income was associated with less AMI cases (p < 0.001 for income-ethnicity interactions).
5.1.3.4 Population-attributable risks
A population-attributable risk perspective (reflecting the prevalence of any one risk factor combined with the strength of the risk it poses) seeks to determine the relative contribution of an observed risk to actual events. Classically, a highly “virulent” risk factor affecting a relatively small number of cases can cause fewer cases when compared to a highly prevalent factor with only marginal risk for converting that risk into an actual event, the latter having a higher population attributable risk for an event. This was indeed observed from a U.S. analysis of the population impact of marginally elevated BP levels derived from the National Health and Nutrition Examination Survey Study [67]. It provides a population target and potential solution (e.g., salt reduction in the food supply chain [68]) beyond the individual level. In this instance, a history of hypertension (30% of AMI cases) was markedly more important than in the global cohort (41.9% versus 23.4%) as a target for preventing de novo AMI. Likewise, abdominal obesity (58.4% versus 33.7%) and less favorable apoB/apoA1 ratios (61.8% versus 46.2%) were also more important contributors to AMI in the African cohort.
5.1.3.5 Cumulative effects of risk factors
Taking into account the cumulative impact of multiple risk factors in the African cohort, a history of diabetes, smoking, and/or hypertension was associated with a population-attributable risk of just under two-thirds of cases (64.5%), with a 17-fold increased risk of presenting as an AMI case compared to controls. When apoB/apoA1 was considered, these values rose to 80.6% and 29-fold, respectively, increasing once again to 89.2% and 49-fold when waist-to-height ratio was additionally considered. Overall, the population-attributable risks for the African cohort were higher than the overall global cohort when all five of these risk factors were assessed. When all nine risk factors of interest were combined (including irregular consumption of fruits/vegetables, no alcohol intake, physical inactivity, and psychosocial stressors) the overall population-attributable risk for de novo AMI in the African cohort related to these factors was 97.4% (i.e., almost all observed cases).
5.1.4 Study interpretation
Beyond aforementioned caveats relating to the geographic location and composition of the study cohort, the African INTERHEART study’s findings strongly suggest that only five risk factors account for almost 9 in 10 de novo presentations of AMI (regardless of ethnicity). As will be discussed in Chapters 6 and 10, while AMI remains relatively rare in those of African ancestry (representing the major portion of the population at risk of future cardiac events) in comparison to high-income countries [9,65,69], the signs are ominous when considering the findings from this research and that outlined in the previous chapter. The INTERHEART investigators concluded that five highly modifiable risk factors indicative of potentially unhealthy lifestyles drove the majority of the AMI patients documented in the global and African-specific component of this landmark study. The major contribution of hypertension to AMI patients is of concern (particularly when considering that CAD and AMI are not the main consequences of undiagnosed/untreated high BP in the African context), as is diabetes.
Key differences according to education and income among the three ethnic groups studied clearly demonstrates that the phenomenon known as epidemiological transition [1–3] is in play. Those of European origin displayed similar patterns of risk according to socioeconomic status as high-income countries [64]. In contrast, it is those of African ancestry who can now “afford” (due to their increased wealth) an unhealthy Westernized diet who appear to be most at risk of AMI. Potential differences in the epidemiological transition of population are also reflected in the pattern of cardiovascular-related case-fatalities. Burden of disease estimates suggest that in South Africa in the year 2000, those of African ancestry had stroke (including hemorrhagic events) and IHD rates of 143/100,000 and 70/100,000, respectively, compared with 72/100,000 and 230/100,000 in those of European origin.
Significantly, there was a clear differential in the median age of the African INTERHEART cohort when compared to the global cohort. Applying to both sexes, the median age of African cases was 4 to 5 years younger overall. Consistent with the overall pattern of noncommunicable forms of heart disease (see Chapter 6), this critical finding suggests that the African population is at particular risk of premature AMI. This may well reflect a pervasive lack of awareness, early detection, effective management, and prevention measures targeting cardiovascular risk factors in vulnerable African communities, noting the relative wealth and resources of South Africa when compared to the remainder of the continent.
Data from the European/other African group reflects an advanced stage of the epidemiological transition as there was found to be a high prevalence rates of cigarette smoking, abdominal obesity, and dyslipidemia, whereas lower prevalence rates of diabetes and hypertension were found. The risk of an elevated apoB/apoA1 ratio was significantly higher in the European/other African group than that found in the global INTERHEART study. Additionally, the most educated and wealthiest segment of the European/other African group had the lowest risk of AMI. This pattern corresponds, for example, to the lower IHD risk factor rates and levels found in the wealthier sector of the population in the UK [70,71]. Their risk profile is also corresponding with the mortality pattern reported above. It is worth noting that the median age of cases in this particular cohort was 54 years, whereas the median age of patients for the global INTERHEART study was 62 years; residually high rates of smoking and dyslipidemia (relative to population changes elsewhere in the world [69]) may well play a significant role in this finding.
The colored African group mainly consisted of individuals from mixed ethnicity ancestry and was predominantly recruited from South Africa. The adoption of Westernization, industrialization, and urbanization among this group is also associated with the epidemiological transition. The recruitment took place at the public sector health services that provided assistance for the poor working class. The risk profile of the controls in this ethnic group nicely illustrates the dangers of adopting potentially hazardous Westernized/industrialized lifestyles. Both men and women were found to have high cigarette smoking habits in addition to often being overweight or obese with parallel increases in the prevalence of hypertension and diabetes in particular. Such a differential in risk is reflected in the estimated rates of CAD and stroke in this ethnic group—higher than those of African ancestry and with much higher rates of stroke than for Europeans.
Overall, the African INTERHEART study’s findings contradicted the prevailing theory (see Chapter 10) that people of African ancestry are essentially “immune” to developing AMI, even when exposed to the type of risk factors [19,72] that drive cases of AMI in the rest of the world. As noted in Chapter 4, reports from various African countries document a changing spectrum and pattern of cardiovascular risk factors (predominantly in urban areas). This must be understood in the context of lifetime exposure to unhealthy lifestyles before these well-known risk factors have a sufficient impact and subjects present with established forms of CVD, particularly relating to atherosclerotic events and noting competing risks for premature mortality in the African context. Supporting this position is the fact that two to three decades ago, African Americans had lower rates of CVD than Caucasian North Americans. However, over time and with extended exposure to risk factors, this has changed, with African Americans demonstrating higher risk of cardiovascular events in the United States [73]. In this context, the risk profile of the Indigenous African group in the African INTERHEART study demonstrates an early stage of epidemiological transition. The study found that there was a relatively small number of AMI cases found among those of African ancestry. However, this may well change given an evolving pattern of risk outlined in Chapter 4. From a more optimistic perspective, these data suggest that AMI in the less-developed areas of sub-Saharan Africa is relatively rare, and in other African countries, the population may be at an even earlier epidemiological transition stage than, for example, South Africa. However, the incremental risk posed by hypertension within those of African ancestry when compared to the global INTERHEART study cohort is of particular concern. As will be highlighted in Chapter 6 and Chapter 13, the impact of highly prevalent hypertension will not only influence de novo cases of AMI but also a broad spectrum of other forms of noncommunicable heart disease without special efforts to control this particular risk factor in those of African ancestry.
5.1.5 Study limitations
As part of the global INTERHEART study, the African-specific findings, outlined above, have the same potential limitations as those reported previously. These include possible confounding if there is differential ascertainment of risk factors between cases and controls, such as possible recall bias when cases of AMI are influenced more because of stress than in controls. The inclusion of patients with first AMI reduces the possibility that individuals with previous CVDs may have altered their lifestyles and risk factor patterns. To minimize bias in the selection of controls, individuals in whom the risk factors of interest in this study were implicated as being protective or harmful were excluded.
5.1.6 Study conclusions
Despite these limitations, it is reasonable to conclude that the African INTERHEART study data revealed that a key set of common risk factors for CVDs accounted for ~90% of case presentations of de novo AMI. As such, the association of five major risk factors for AMI in the African population is consistent with that found in the global INTERHEART study cohort. Fortunately, these data also confirm that those of African ancestry are in early epidemiological transition toward noncommunicable forms of heart disease and specifically AMI. There is still time, therefore, to invest in lifestyle modification, primary prevention, early diagnosis, rapid-access treatment options, and secondary prevention strategies, adapted to the African context, in order to prevent a future epidemic of AMI and to minimize its potentially devastating consequences if a primary event should occur.