CHAPTER 7
Rheumatic heart disease
Simon Stewart1, Melinda Jane Carrington1, and Karen Sliwa2
1 Australian Catholic University, Melbourne, Victoria, Australia
2 University of Cape Town, South Africa; University of the Witwatersrand, Johannesburg, South Africa
7.0 Introduction
As outlined in more detail in Section 2, RHD with its close link to childhood poverty and heavy exposure to communicable diseases (in this case acute rheumatic fever) represents one of the truly reliable barometers of the socioeconomic wealth of a population and country, particularly in the setting of limited access to even basic preventive health care measures. The poorer the country, the higher the rate of childhood cases of RHD, with often deadly consequences. Beyond clearly identifiable index childhood cases, however, there is undoubtedly a “legacy effect” of acute rheumatic fever (estimated to affect millions of people across the globe)—the subsequent development of subclinical RHD that may well reveal itself in adulthood as increasingly significant valve disease/dysfunction. Certainly, this is most probably the case even in high-income countries among individuals who were exposed to childhood poverty but survived to a very advanced age. Derived from the main results of the Heart of Soweto Study (see Chapter 6), it was postulated that the adult population in Soweto would be particularly vulnerable to this otherwise poorly researched and reported clinical phenomenon.
7.1 A legacy effect of RHD from childhood to adulthood in an urban African community
Sliwa K, Carrington M, Mayosi B, Zigriades E, Mvungi R, Stewart S. Incidence and characteristics of newly diagnosed rheumatic heart disease in urban African adults: insights from the Heart of Soweto Study. European Heart Journal 2010; 31(6):719–27. [3]
7.1.1 Study background
Worldwide it is estimated that almost 16 million people per annum are affected by RHD as a consequence of streptococcal infection with subsequent acute rheumatic fever and involvement of the cardiac valves as part of a systemic inflammatory response/process [1,2]. Consequently, it has been estimated that approximately 200,000 deaths per annum among those living in low-income countries are attributable to RHD [3]. As with most forms of communicable disease, the peoples of sub-Saharan Africa are particularly vulnerable to RHD. However, as these (global) figures largely relate to children and young adults, they are likely to underestimate the true burden of RHD as reflected in the adult population (via the legacy effect described above). As described in Chapter 6, the Heart of Soweto Study cohort provided a perfect opportunity to explore the balance between communicable and noncommunicable forms of heart disease in a population in epidemiological transition.
7.1.2 Study aims
Using systematic clinical profiling of the Heart of Soweto Study cohort to investigate de novo presentations of heart disease, with a careful examination and classification of all cases of valvular disease and dysfunction via echocardiography, this study focused on the probable incidence and clinical characteristics of RHD in the Sowetan community.
7.1.3 Study methods
Overall, 960 patients presenting with valvular abnormalities to the Cardiology Unit at the Chris Hani Baragwanath Hospital during the period 2006 to 2007 and enrolled in the Heart of Soweto Study (see Chapter 6 for detailed study methods and the spectrum of disease presentation) were subject to further clinical investigation and scrutiny as part of this study report. As described in the original report, classifying inherently complex cases of valve disease and dysfunction in this cohort was both challenging and problematic. However, this resulted in the most comprehensive report of the clinical spectrum of valve disease in sub-Saharan Africa (at least from an adult perspective) to that point.
As described in the original report, RHD was diagnosed from a history of acute rheumatic fever and/or a cardiac murmur plus standard echocardiographic criteria [4]; the latter included detection of typical bowing or doming of the mitral valve leaflets in diastole. Similarly, mitral stenosis was diagnosed on the basis of thickening or calcification of the leaflets (typically the posterior leaflet with subvalvular region involvement). Isolated or concomitant MR was diagnosed by any definitive evidence of regurgitation seen in two planes by Doppler evaluation using semiquantitative measures, while severe MR was detected from additional systolic flow reversal in the pulmonary veins.
7.1.4 Study findings
7.1.4.1 Newly diagnosed VHD
Of 4,005 de novo presentations to the Cardiology Unit of the Chris Hani Baragwanath Hospital during the study period, 960 (24.0%) had a valvular abnormality. Affected patients were predominantly of African ancestry (n = 868 [90.4%]), women (n = 570 [59.4%]), and long-term residents of Soweto (n = 512 [53.3%]). Overall, there was an equal balance between structural VHD (n = 481 [50.1%]), and functional VHD (n = 439 [45.7%]). As shown in Table 7.1, the most common structural valve disease presented was RHD, found in 344 (71.5%) patients. Other structural abnormalities included CHD (n = 20; 55.6%), mitral valve prolapse (n = 5; 13.9%), myxomatous mitral valve (n = 3; 8.3%), submitral aneurysm (n = 2; 5.6%), Marfan syndrome (n = 1; 2.8%), subaortic aneurysm (n = 12.8%), hypertrophic CMO (n = 1; 2.8%), and unclassified (n = 3; 8.3%).
Table 7.1 Abnormal presentations of structural valve disease according to sex.
| Structural Valve Disease Abnormality | Women (n = 325) | Men (n = 156) | All (n = 481) |
| Degenerative | 65 (20.0%) | 36 (23.1%) | 101 (21.0%) |
| RHD | 234 (72.0%) | 110 (70.5%) | 344 (71.5%) |
| Other structural abnormalities | 26 (8.0%) | 10 (6.4%) | 36 (7.5%) |
| CHD | 15 (57.7%) | 5 (50.0%) | 20 (55.6%) |
| Hypertrophic CMO | 0 (0%) | 1 (10.0%) | 1 (2.8%) |
| Marfan syndrome | 1 (3.8%) | 0 (0%) | 1 (2.8%) |
| Mitral valve prolapse | 3 (11.5%) | 2 (20.0%) | 5 (13.9%) |
| Myxomatous mitral valve | 3 (11.5%) | 0 (0%) | 3 (8.3%) |
| Subaortic aneurysm | 1 (3.8%) | 0 (0%) | 1 (2.8%) |
| Submitral aneurysm | 1 (3.8%) | 1 (10.0%) | 2 (5.6%) |
| Unclassified | 2 (7.7%) | 1 (10.0%) | 3 (8.3%) |
Figure 7.1 shows the pattern of structural valve disease abnormalities in this cohort according to sex with roughly similar patterns of presentation on this basis. Similarly, Figure 7.2 shows the pattern of disease associated with functional valve dysfunction according to sex. From this perspective, women were more likely to present with valvular dysfunction associated with hypertensive heart disease (OR 1.70, 95% CI 1.03–2.81; p = 0.042) and dilated CMO (OR 1.52, 95% CI 1.07–2.18; p = 0.026). Alternatively, men were more likely to present with RHF/cor pulmonale (OR 2.11, 95% CI 1.54–2.90; p < 0.0001) and concurrent ischemic CMO (OR 3.56, 95% CI 1.62–7.88; p = 0.026).
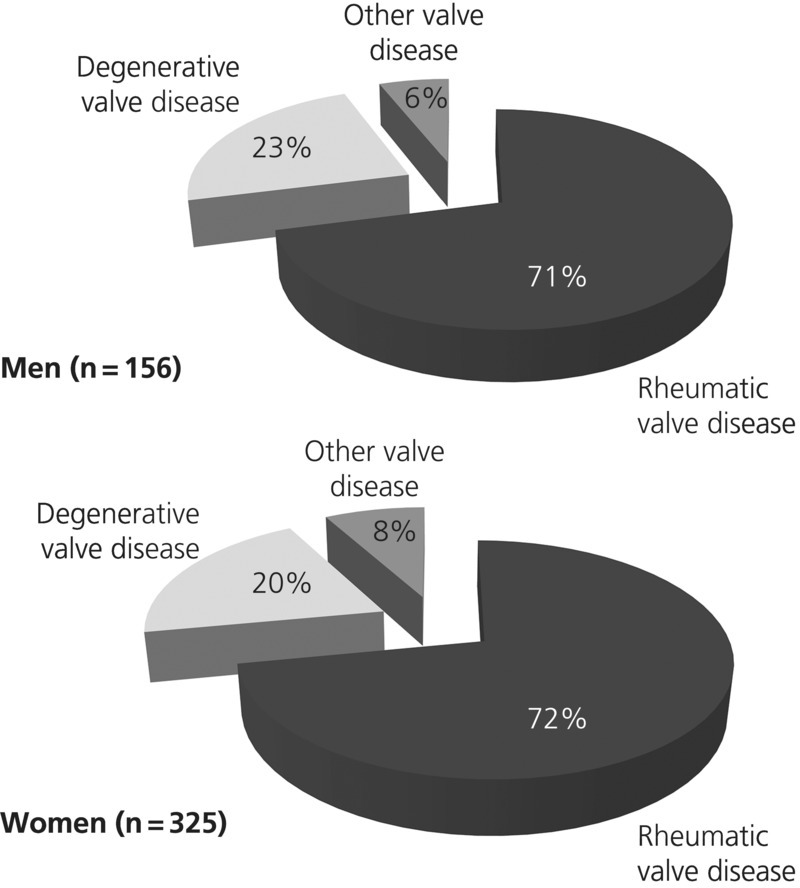
Figure 7.1 The structural valve disease pattern according to sex.
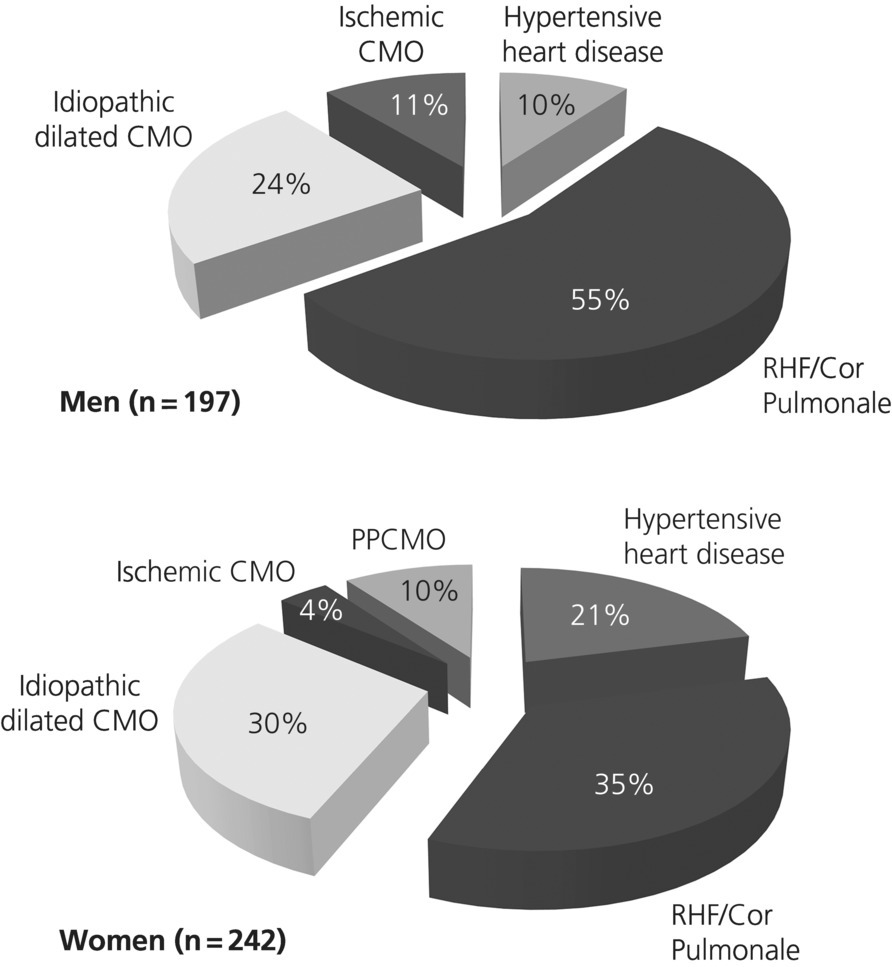
Figure 7.2 Functional valve dysfunction according to sex.
7.1.4.2 Newly diagnosed RHD patients
Overall, the patients presenting with newly diagnosed RHD were predominantly women of African ancestry (n = 234; 68.0%), and the prevalence pattern appeared to increase in the economically productive age group before declining again (i.e., age groups 14 to 19 years [3%], 20 to 29 years [16%], 30 to 39 years [24%], 40 to 49 years [17%], 50 to 59 years [19%], 60 to 69 years [12%], and >70 years [8%]). Men were more likely to present in NYHA Class III/IV (20.9% versus 16.2%) when compared to women. Alternatively, women were more likely to present with dyspnea (68.0% versus 60.9%) and peripheral edema (29.5% versus 22.7%). Women also presented with higher heart rates (86 ± 18 versus 81 ± 16) while having similar BP profiles (see Table 7.2).
Table 7.2 Patients’ demographic and clinical characteristics and clinical presentation.
| Women (n = 234) | Men (n = 110) | All (n = 344) | |
| Demographic characteristics | |||
| African ancestry | 220 (94.0%) | 94 (85.5%) | 314 (91.3%) |
| Median age in years | 41 (30–55) | 42 (31–55) | 43 (32–56) |
| Median years in Soweto | 35 (20–49) | 37 (20–50) | 36 (20–50) |
| Standard education, 0–10 years | 213 (91.0%) | 101 (91.8%) | 314 (91.3%) |
| Women | 234 (100%) | 0 (0%) | 234 (68.0%) |
| Clinical characteristics | |||
| Anemia | 23 (9.8%) | 4 (3.6%) | 27 (7.9%) |
| AF | 20 (8.6%) | 14 (12.7%) | 34 (9.9%) |
| Renal dysfunction | 28 (12.0%) | 29 (26.4%) | 57 (16.7%) |
| Clinical presentation | |||
| Heart rate, beats/min | 86 ± 18 | 81 + 16 | 84 + 17 |
| Systolic BP, mm Hg | 122 ± 24 | 121 + 24 | 122 + 24 |
| Diastolic BP, mm Hg | 69 ± 21 | 67 ± 18 | 69 ± 20 |
| Dyspnea on presentation | 159 (67.9%) | 67 (60.9%) | 226 (65.7%) |
| Palpitations/chest pain | 156 (66.7%) | 71 (64.6%) | 227 (65.9%) |
| Peripheral edema | 69 (29.5%) | 25 (22.7%) | 94 (27.3%) |
| Raised jugular venous pressure | 12 (5.1%) | 7 (6.4%) | 19 (5.5%) |
| NYHA III/IV | 38 (16.2%) | 23 (20.9%) | 61 (17.7%) |
As shown in Table 7.3, MR was revealed as the most common valvular lesion (affecting 59% of the patients) and was commonly associated with left and right heart abnormalities. Alternatively, aortic stenosis was the least common valvular lesion (9% of cases).
Table 7.3 Patients’ RHD echocardiographic presentations according to predominant valvular lesion, and prescribed medication.
| Aortic Regurgitation (n = 126) | Aortic Stenosis (n = 31) | Mitral Regurgitation (n = 204) | Mitral Stenosis (n = 103) | All (n = 344) | |
| Systolic function | |||||
| LV diameter systole (mm) | 37 ± 14 | 31 ± 14 | 38 ± 31 | 33 ± 13 | 36 ± 21 |
| LV diameter systole >45 mm | 22 (17.5%) | 3 (9.7%) | 27 (13.2%) | 8 (7.8%) | 55 (16.0%) |
| LV diameter diastole (mm) | 51 ± 14 | 49 ± 14 | 51 ± 11 | 44 ± 10 | 49 ± 12 |
| LV diameter diastole >55 mm | 40 (31.8%) | 6 (19.4%) | 49 (24.0%) | 7 (6.8%) | 86 (25.0%) |
| LVEF (%) | 56 ± 14 | 60 ± 13 | 58 ± 13 | 59 ± 11 | 58 ± 13 |
| LV systolic dysfunction | 23 (18.3%) | 7 (22.6%) | 28 (13.7%) | 11 (10.7%) | 50 (14.5%) |
| Valvular regurgitation (mild-moderate-severe) | |||||
| Aortic valve | 45-37-18 | 35-32-13 | 12-8.3-3.4 | 12-6.8-0 | 126 (36.6%) |
| Mitral valve | 22-8.7-7.1 | 19-6.5-3.2 | 43-40-17 | 20-17-4.9 | 103 (29.9%) |
| Tricuspid valve | 9.5-9.5-6.4 | 13-3.2-3.2 | 13-9.3-7.4 | 8.7-14-11 | 89 (25.9%) |
| Other abnormalities | |||||
| RV systolic pressure >35 mm Hg | 19 (15.1%) | 3 (9.7%) | 40 (19.6%) | 26 (25.3%) | 62 (18.0%) |
| Prescribed medication | |||||
| Loop diuretics | 76 (60.3%) | 22 (71.0%) | 116 (56.9%) | 69 (67.0%) | 208 (60.5) |
| Antiplatelet therapy | 50 (39.7%) | 12 (38.7%) | 67 (32.8%) | 45 (43.7%) | 130 (37.8%) |
| ACE-inhibitor | 45 (35.7%) | 10 (32.3%) | 53 (26.0%) | 26 (25.2%) | 94 (27.3%) |
| Calcium antagonist | 28 (22.2%) | 6 (19.3%) | 16 (7.8%) | 6 (5.8%) | 39 (11.3%) |
| Beta-blocker | 24 (19.1%) | 9 (29.0%) | 44 (21.6%) | 42 (40.8%) | 89 (25.9%) |
| Aldosterone inhibitor | 20 (18.9%) | 6 (19.3%) | 28 (13.7%) | 13 (12.6%) | 47 (13.7%) |
| Digoxin | 16 (12.7%) | 3 (9.7%) | 24 (11.8%) | 14 (13.6%) | 43 (12.5%) |
7.1.4.3 Outcomes
Overall, 90 (26.2%) patients with RHD were admitted to hospital for suspected bacterial endocarditis ≤30 months after diagnosis. Additionally, 75 (21.8%) patients were referred for aortic and mitral valve replacement (22 [29.3%] patients and 32 [42.7%] patients, respectively) and 21 (28%) patients for double valve replacement.
7.1.4.4 Estimated incidence of RHD
These hospital data permitted some estimates of the incidence of RHD in those aged 14 years or more living in the surrounding area of Soweto (on age and sex-specific basis). Overall, it was estimated that, annually, there are ~24 new cases of RHD per 100,000 in Soweto. However, a j-shaped distribution of incident cases was highly evident with incidence rates rising from 15 to 53 cases per 100,000 per annum in those aged between 19 and 60 years (the highest point being in older cases) following an initial (high) rate of 30 cases per 100,000 per annum among those aged 15 to 19 years.
7.1.5 Study interpretation
As originally reported, this was the first study to examine the incidence of RHD among those of African ancestry aged >14 years [5]. Overall, these data revealed that RHD remains common among the studied adult population, challenging the long-held assumption that RHD predominantly affects children (particularly in populations in epidemiologic transition) [6]. Significantly, these data suggest that RHD principally affects individuals in economically productive age groups in Soweto (particularly those aged 30–39 years). Furthermore, primary VHD plays a vital role in the development of HF in the sub-Saharan African context. Early detection of valvular disease and dysfunction is clearly crucial in this context. However, the challenge of assessing patients with cardiac murmurs in LMIC remains. In total, 960 out of 4,005 de novo patients presented to the cardiac unit with a valvular abnormality (more than half of cases presenting with functional VHD). In developed countries, VHD is not considered a concern and is often overlooked in terms of morbidity and mortality [7]. Numerous reports on the investigation of functional MR in ischemic and nonischemic CMOs have been published, as well as their contribution to the burden of HF [8,9].
7.1.6 Study limitations
A number of limitations were noted throughout this study. First, the study sample included only individuals who were privileged enough to seek professional medical care, and these patients usually presented with advanced forms of valvular heart disease. Therefore, this hospital-based study likely underrepresented the true portion of the population of Soweto affected by the full spectrum of valve disease and dysfunction. Unfortunately, given the nature and context of the study population, follow-up of study patients was limited and the long-term impact and natural history of RHD and other forms of valve disease and dysfunction in this and other sub-Saharan African patient populations remains largely unknown.
7.1.7 Study conclusions
As noted in the original report, this complex clinical picture of valvular disease and dysfunction required careful interpretation, including a new standard for classifying cases. Quite apart from a unique report on late-stage RHD, this pivotal study uncovered a significant component of valve disease and dysfunction as part of late presentations of heart disease in Soweto. Given the volume of cases derived from the systematic application of echocardiography, there is a clear mandate to explore novel and cost-effective methods (e.g., handheld echocardiography and automated heart sound interpretation) to discover otherwise undiagnosed valve disease/dysfunction and implement preventive treatments.