CHAPTER 8
Pericardial disease
Mpiko Ntsekhe
University of Cape Town/Groote Schuur Hospital, Cape Town, South Africa
8.0 Introduction
Inflammation, infection, and other noxious insults to the thin-layered membrane surrounding the heart are capable of triggering a range of responses within the pericardium, which often culminate in clinically evident disease of the pericardium. Traditionally, pericardial diseases are classified by mode of clinical presentation into the following: (a) acute pericarditis with or without pericardial effusion, (b) effusive pericarditis with or without tamponade (c) effusive constrictive pericarditis, and (d) constrictive pericarditis [10]. Pericardial disease is a relatively common cause of cardiac morbidity and mortality in sub-Saharan Africa [11]. In THESUS-HF, pericardial disease was as common a cause of HF as IHD (both approximately 7%) [12] (see Section 5). These results were similar to findings from the large Heart of Soweto Study, which was designed to ascertain the spectrum of heart disease among a prospective cohort of patients referred to one of Africa’s largest hospitals for cardiac evaluation [13]. The predominant clinical presentations of pericardial disease in parts of Asia and in sub-Saharan Africa, where TB is endemic and coinfection with HIV is common, are effusive pericarditis with or without tamponade and constrictive pericarditis [14]. Tuberculous pericarditis results in high morbidity and mortality rates despite anti-TB therapy, pericardial drainage, or pericardiectomy [15–17]. Evidence from early studies of patients with tuberculous pericarditis showed that glucocorticoid therapy may improve outcomes and decrease mortality by reducing pericardial constriction and cardiac tamponade. A meta-analysis comprising two randomized controlled trials examining glucocorticoid therapy for tuberculous pericarditis reported a nonsignificant reduction in mortality rates [18,19]. However, these studies were limited by small sample sizes and number of events recorded [18–20]. Preliminary data from a number of sources provide a potential mechanism via which adjunctive immunotherapy may improve outcomes in tuberculous pericarditis. This evidence indicates that inflammation associated with TB may be reduced by corticosteroids while repeated doses of intradermal heat-killed Mycobacterium indicus pranii may increase CD4+ T-cell counts in HIV-positive patients and alter phenotype of the immune response within the pericardium [21–23]. However, a limitation of therapy is data suggesting the potential for an increased risk of cancer in HIV-infected patients [24,25]. It was on this basis that Mayosi and colleagues conducted the Investigation of the Management of Pericarditis (IMPI) randomized controlled trial of the efficacy and safety of adjunctive immunotherapy for patients with tuberculous pericarditis [26].
8.1 Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis
Mayosi BM, Ntsekhe M, Bosch J, Pandie S, Jung H, Gumedze F, Pogue J, Thabane L, Smieja M, Francis V, Joldersma L, Thomas KM, Thomas B, Awotedu AA, Magula NP, Naidoo DP, Damasceno A, Banda AC, Brown B, Manga P, Kirenga B, Mondo C, Mntla P, Tsitsi JM, Peters F, Essop MR, Russell JBW, Hakim J, Matenga J, Barasa AF, Sani MU, Olunuga T, Ogah O, Ansa V, Aje A, Danbauchi S, Ojji D, Yusuf S. Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. New England Journal of Medicine 2014; 371(12):1121–30.
8.1.1 Study aims
This study evaluated the efficacy and safety of adjunctive prednisolone and Mycobacterium indicus pranii in African patients who had tuberculous pericarditis with or without concurrent HIV. It was hypothesized that adjunctive prednisolone (by suppressing intrapericardial inflammation), or intradermal Mycobacterium indicus pranii (by altering the cellular phenotype of the local immune response) would be of overall benefit with regard to major adverse pericardial disease events. A two-by-two factorial design was conducted to measure intradermal Mycobacterium indicus pranii and prednisolone for the treatment of tuberculous pericarditis compared with placebo. The study drugs used were donated and distributed to the research sites by Cadila Pharmaceuticals. The protocol was reviewed by the Canadian Institutes of Health Research and the South African Medical Research Council. Ethics approval was obtained at each participating site along with informed consent from all patients.
8.1.2 Study methods
8.1.2.1 Patient enrollment
Patients included in the study had pericardial effusion confirmed by echocardiography, had probable or definite tuberculous pericarditis, had been receiving anti-TB treatment for less than one week before enrollment, and were >18 years of age. Patients were excluded from the study if they had used glucocorticoids within the last 30 days, if they were pregnant, allergic, or hypersensitive to Mycobacterium indicus pranii preparation, or if another cause of pericardial disease was identified.
8.1.2.2 Study procedures
Patients were randomized into either prednisolone or Mycobacterium indicus pranii groups and both were compared with two independent placebo groups. With regard to the prednisolone comparison, placebo and prednisolone were given to the different comparison groups for 6 weeks. A dose of 120 mg was administered daily in the first week, 90 mg daily in the second week, 60 mg daily for the third week, 30 mg daily for the fourth week, 15 mg daily for the fifth week, and 5 mg daily for the last week. With regard to the Mycobacterium indicus pranii comparison, the placebo and the Mycobacterium indicus pranii were administered to both groups in five doses of 0.1 mL. The first dose was administered at baseline by a single injection into the deltoid muscle region of the upper arm, three successive injections were administered at the same site at 2 week intervals, and the remaining fifth dose administered at 3 months. HAART for HIV and antimicrobial treatment for TB was given to the trial patients according to the WHO guidelines [29–32]. No routine testing for drug resistance of either Mycobacterium TB isolates or HIV isolates was performed before or during treatment. Follow-up data collection comprised assessments of study outcomes (details in section below), recording of adverse events, and treatment adherence. These data were collected at the time of hospital discharge; at 2, 4, and 6 weeks; at 6 months; and then every 6 months for 2 years and every 12 months thereafter over the 5-year study period. Site monitoring throughout the study was performed through the project coordinating office according to a standard operating procedure.
8.1.2.3 Outcomes and statistical analysis
The primary efficacy outcome in the study was the composite of death, the first occurrence of cardiac tamponade requiring pericardiocentesis, or the development of constrictive pericarditis. The secondary efficacy outcomes were hospitalization as well as the individual components of the primary outcome. Cancer, opportunistic infections, CD4+ T-lymphocyte cell count, and the incidence of immune reconstitution inflammatory syndrome (in those with HIV) comprised the safety outcomes. The sample size calculation was based on the following assumptions: that the event rate among patients receiving placebos for both interventions would be 35% at a mean follow-up of 2 years, the rate of loss to follow-up would be 6%, and approximately 50% of the patients in each intervention’s placebo group would receive an effective intervention that would result in 30% relative risk reduction in the event rate. Based on this, a sample size of 1,400 patients would result in 90% power to detect a 22.9% reduction in the hazard ratio, with use of a log-rank test and a two-sided type I error rate of 5%.
8.1.3 Study findings
8.1.3.1 Study population
The trial was conducted during the period January 2009 to February 2014. Patients were recruited from 19 hospitals in 8 African countries (South Africa, Mozambique, Malawi, Uganda, Zimbabwe, Sierra Leone, Kenya, and Nigeria). Overall, a total of 1,400 patients were recruited. These patients were randomized into either the placebo (n = 694; 49.6%) or the prednisolone (n = 706; 50.4%) group for 6 weeks (see Figure 8.1). Median follow-up period was 637 days. Primary outcome status was known for 1,371 (97.9%) of these patients. An additional 1,250 patients were randomized into either the placebo (n = 625; 50%) or the Mycobacterium indicus pranii (n = 625; 50%) group (Figure 8.1). Median follow-up period was 721 days. Primary outcome status was known for 1,223 (97.8%) of these patients.
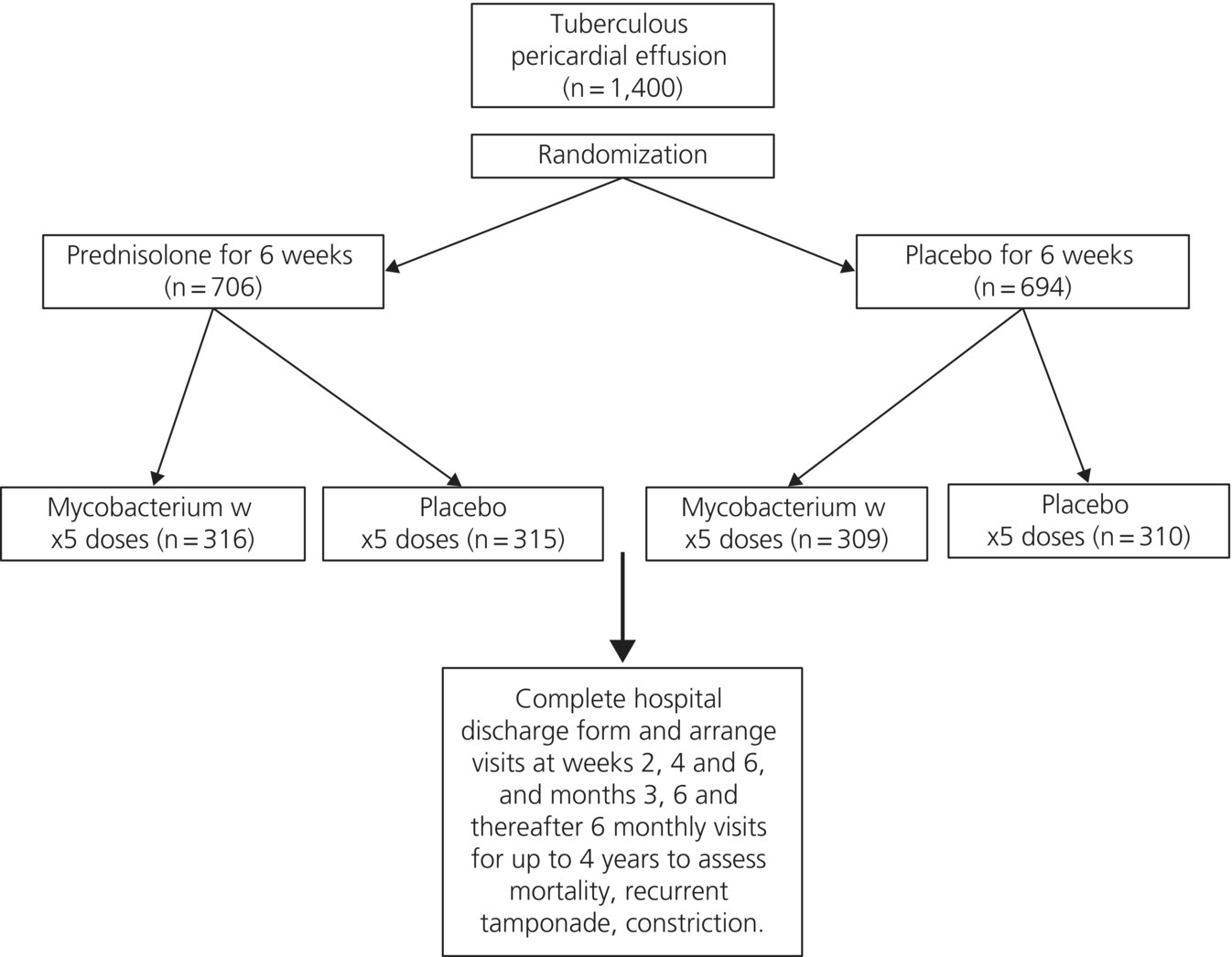
Figure 8.1 Trial study design.
Baseline characteristics of all those who participated in the study are presented in Table 8.1 with a similar profile evident according to group assignment. Both groups had more male than female patients. Approximately two-thirds had a large pericardial effusion, and pericardiocentesis was performed in over 60% of the patients. The diagnosis of tuberculous pericarditis was confirmed in 17.1% of patients, and over two-thirds were found to be HIV positive.
Table 8.1 Patients’ baseline, 3-month follow-up, and outcome characteristics.
| Mycobacterium indicus pranii Group (n = 625) | Placebo Group (n = 625) | Prednisolone Group (n = 706) | Placebo Group (n = 694) | |
| Age (years) | 37.7 ± 12.5 | 39.3 ± 14.1 | 38.8 ± 13.5 | 38.5 ± 13.3 |
| Men | 333 (53.3%) | 362 (57.9%) | 389 (55.1%) | 395 (56.9%) |
| Weight (kg) | 58.6 ± 12.2 | 59.6 ± 12.0 | 59.6 ± 12.3 | 59.2 ± 12.1 |
| Diagnosis at 3-Months | ||||
| Definite Tuberculous Pericarditis | 100 (16.0%) | 105 (16.8%) | 116 (16.4%) | 122 (17.6%) |
| Probable Tuberculous Pericarditis | ||||
| TB Proven Elsewhere | 67 (10.7%) | 53 (8.5%) | 73 (10.3%) | 63 (9.1%) |
| TB Not Proven Elsewhere | 450 (72.0%) | 462 (73.9%) | 506 (71.7%) | 506 (72.9%) |
| Non-tuberculous cause | 8 (1.3%) | 5 (0.8%) | 11 (1.6%) | 3 (0.4%) |
| HIV-status | ||||
| Positive | 437 (69.9%) | 403 (64.5%) | 474 (67.1%) | 465 (67.0%) |
| Negative | 175 (28.0%) | 209 (33.4%) | 218 (30.9%) | 213 (30.7%) |
| Unknown | 13 (2.1%) | 13 (2.1%) | 14 (2.0%) | 16 (2.3%) |
| Pericardiocentesis | ||||
| Performed | 372 (59.5%) | 381 (61.0%) | 428 (60.6%) | 419 (60.4%) |
| Not performed | 253 (40.5%) | 244 (39.0%) | 278 (39.4%) | 275 (39.6%) |
| Size of Pericardial Effusion | ||||
| Small, < 1 cm | 58 (9.3%) | 40 (6.4%) | 51 (7.2%) | 56 (8.1%) |
| Moderate, 1-2 cm | 154 (24.6%) | 140 (22.4%) | 172 (24.4%) | 159 (22.9%) |
| Large, > 2 cm | 391 (62.6%) | 428 (68.5%) | 462 (65.4%) | 460 (66.3%) |
| Not Measured | 22 (3.5%) | 17 (2.7%) | 21 (3.0%) | 19 (2.7%) |
| HAART | 88 (14.1%) | 84 (13.4%) | 99 (14.0%) | 104 (15.0%) |
| Anti-TB Therapy | 460 (73.6%) | 462 (73.9%) | 541 (76.6%) | 531 (76.5%) |
| Primary Composite Outcomes | 156 (25.0%) | 152 (24.3%) | 168 (23.8%) | 170 (24.5%) |
| Secondary Outcomes | ||||
| Cardiac Tamponade | 22 (3.5%) | 22 (3.5%) | 22 (3.1%) | 28 (4.0%) |
| Constrictive Pericarditis | 36 (5.8%) | 37 (5.9%) | 31 (4.4%) | 54 (7.8%) |
| Death From Any Cause | 119 (19.0%) | 111 (17.8%) | 133 (18.8%) | 115 (16.6%) |
| Hospitalisation | 152 (24.3%) | 141 (22.6%) | 146 (20.7%) | 175 (25.2%) |
8.1.3.2 Treatment regimens and adherence
With regard to the comparison of placebo with prednisolone, 88.7% of the patients in the placebo group and 88.5% of those in the prednisolone group adhered to the regimen for the full study treatment of 6 weeks. A total of 44 (3.1%) patients received nonstudy glucocorticoids during the trial, and this rate was similar in the placebo and prednisolone groups. Adherence was slightly lower in the Mycobacterium indicus pranii and associated placebo group. A total of 81.4% of the patients in the placebo group and 75.9% of those in the Mycobacterium indicus pranii group adhered to the regimen for the full study treatment of 3 months. Of the 1,400 Prednisolone comparison patients enrolled in the trial, 14.5% were receiving HAART and 76.6% were receiving anti-TB treatment at the time of randomization. The growing use of HAART during the course of the trial is attributed to revised WHO guidelines recommending early HAART initiation in HIV-positive patients presenting with TB.
8.1.3.3 Prednisolone comparison
The rate of the primary composite outcome (cardiac tamponade requiring pericardiocentesis, constrictive pericarditis, or death) was 14.3 events per 100 person-years of follow-up in the prednisolone group and 14.8 per 100 person-years in the placebo group. When considered individually, there were no significant differences between groups in the rate of cardiac tamponade requiring pericardiocentesis or in the rate of death. The main causes of death were determined to be disseminated TB (18.6%), HIV infection (7.3%), pericarditis (23.8%), and other cardiovascular causes (5.7%). The placebo group had more hospitalizations and a higher rate of constrictive pericarditis than the prednisolone group. Table 8.2 shows the effects of prednisolone and Mycobacterium indicus pranii immunotherapy on safety outcomes. The incidence of opportunistic infection was 6.89 cases per 100 patient-years in the prednisolone group as compared with 5.91 per 100 patient-years in the placebo group. The proportion of patients with candidiasis, cancer, and HIV-related cancer was significantly higher in the prednisolone group when compared to the placebo group. Prednisolone was associated with an increased incidence of cancer relative to placebo. This increase was due to a higher incidence of HIV-related cancers in the prednisolone group. A list of the causes of cancer is provided in Table 8.3.
Table 8.2 Effects of prednisolone and Mycobacterium indicus pranii immunotherapy on safety outcome.
| Prednisolone (n = 706) | Placebo (n = 694) | Mycobacterium indicus pranii (n = 625) | Placebo (n = 625) | |||||
| Outcome | No. of patients | No. of events/100 person-yr | No. of patients | No. of events/100 person-yr | No. of patients | No. of events/100 person-yr | No. of patients | No. of events/100 person-yr |
| Cancer | 13 (1.8%) | 1.05 | 4 (0.6%) | 0.32 | 11 (1.8%) | 0.92 | 3 (0.5%) | 0.24 |
| Candida infection | 54 (7.6%) | 4.68 | 36 (5.2%) | 3.01 | 47 (7.5%) | 4.17 | 37 (5.9%) | 3.20 |
| HIV-related cancer | 9 (1.3%) | 0.73 | 1 (0.1%) | 0.08 | 7 (1.1%) | 0.58 | 2 (0.3%) | 0.16 |
| Immune reconstitution disease | 2 (0.3%) | 0.16 | 1 (0.1%) | 0.08 | 1 (0.2%) | 0.08 | 1 (0.2%) | 0.08 |
| Opportunistic infection | 78 (11%) | 6.89 | 68 (9.8%) | 5.91 | 75 (12%) | 6.86 | 61 (9.8%) | 5.45 |
Table 8.3 The causes of malignancy according to the prednisolone comparison.
| Prednisolone (n = 706) | Placebo (n = 649) | |
| All malignancies | 13 (1.8%) | 4 (0.6%) |
| Gastric/colon cancer | 1 (0.1%) | 0 (0%) |
| Kaposi | 7 (1%) | 1 (0.1%) |
| Non-Hodgkin’s lymphoma | 2 (0.3%) | 0 (0%) |
| Progression of preexisting malignancy | 1 (0.1%) | 1 (0.1%) |
| Other | 2 (0.3%) | 2 (0.3%) |
8.1.3.4 Mycobacterium indicus pranii comparison
The primary composite outcome rates and components, as well as the rates of opportunistic infection and hospitalization, did not differ significantly between the Mycobacterium indicus pranii group and the placebo group. However, as compared with placebo, Mycobacterium indicus pranii was significantly associated with an increased cancer incidence (0.92 versus 0.24 cases per 100 person-years; p = 0.03), which was mainly due to an increase in HIV-related cancer (shown in Tables 8.2 and 8.3). There was a similar nonsignificant difference in the increase in CD4+ T-cell counts, HIV-related cancer, and one immune reconstitution inflammatory syndrome case in both groups. For obvious reasons, patients in the placebo group had fewer injection-site reactions than those in the Mycobacterium indicus pranii group. While the majority of these reactions were characterized by inflammation signs and minor symptoms, there was a significantly greater proportion of patients with abscess formation (15% versus 1%, p < 0.001) in the Mycobacterium indicus pranii group than in the placebo group. These injection-site side effects negatively affected adherence to the treatment (21% nonadherence) in the Mycobacterium indicus pranii group.
8.1.3.5 Prednisolone and Mycobacterium indicus pranii interaction and subgroup analysis
There was no significant interaction between the effects of prednisolone and Mycobacterium indicus pranii on the primary efficacy and safety outcomes (p > 0.30), except for injection-site reactions (p = 0.004). However, 9 of the 13 cancer cases in the prednisolone group occurred in patients who also received Mycobacterium indicus pranii. The number of cases is small, although a clinical interaction of the two interventions on cancer cannot be ruled out. As identified by forest plots, the effects of Mycobacterium indicus pranii immunotherapy and prednisolone therapy on the primary composite efficacy outcome were similar across the key subgroups (prespecified above).
8.1.4 Study interpretation
Overall, neither Mycobacterium indicus pranii immunotherapy nor prednisolone therapy had a significant effect on reducing the primary composite outcome of constrictive pericarditis, cardiac tamponade requiring pericardiocentesis, or mortality. Adjunctive prednisolone therapy decreased the incidence of hospitalization and constrictive pericarditis. However, both interventions increased the incidence of HIV-related cancer among trial patients. The trial had a number of strengths. First, a large cohort of 1,400 patients, 940 of whom were HIV positive, were recruited into the trial, providing a robust platform for testing the study hypotheses. Second, beyond examining treatment efficacy, treatment adherence and adverse events were carefully monitored and analyzed. Previous trials of adjunctive glucocorticoid therapy in those with tuberculous pericarditis were relatively small, included few HIV-infected patients, and provided little detail on potential adverse events. Most important, this was the first study to examine Mycobacterium indicus pranii immunotherapy in this patient population. A number of additional aspects to this study require comment. A dose of 120 mg per day of prednisolone was administered at baseline, which is identified to have a therapeutic effect when administered in combination with rifampin, an enzyme inducer that increases the metabolism of glucocorticoids. Throughout the study, prednisolone therapy adherence was high. The doses used throughout the 6-week period were sufficient to achieve a substantial anti-inflammatory effect, as there was a significant reduction in pericardial constriction. The reduction in the incidence of constrictive pericarditis also translated to fewer hospitalizations in the prednisolone-treated group. This is important because pericardiectomy is associated with high perioperative morbidity and mortality, and cardiac surgery is not widely available in many parts of sub-Saharan Africa with limited health care resources. The study also revealed a positive association between prednisolone therapy and HIV-related cancer. These results are consistent with two previous studies of HIV-associated TB, in which cases of Kaposi’s sarcoma occurred only in the prednisolone-treated groups [24,25]. However, the association of HIV-related cancer with Mycobacterium indicus pranii immunotherapy observed in this study has not been reported previously. It is possible that adjunctive glucocorticoids and Mycobacterium indicus pranii act synergistically to increase the risk of cancer in immunosuppressed patients. Unfortunately, the available data on the interaction between Mycobacterium indicus pranii immunotherapy and adjunctive glucocorticoid therapy are limited [26].
8.1.5 Study limitations
This study had a number of limitations that also require comment. First, a diagnosis of TB either in the pericardium or elsewhere in the body was made in only one-quarter of the patients. This is because the diagnosis of extrapulmonary TB is challenging, and only a small proportion of such cases are treated on the basis of a definite diagnosis. Thus, one interpretation of the results may be that the intervention was ineffective due to the limited number of patients with diagnosed tuberculous pericarditis. However, this is unlikely as the results for those with definite TB and those with probable TB were consistent. Second, although the estimation of the sample size needed for this study was based on the clinical case definition of tuberculous pericarditis, it was expected that a small proportion of cases would have an alternative cause of pericarditis. This was indeed the case, with a small proportion (less than 2%) of patients having a diagnosis other than TB. Third, the trial was powered for a rate of nonadherence of 10% in the active-treatment groups. Although this rate was almost achieved in the prednisolone group (11%), the nonadherence rate was 21% in the Mycobacterium indicus pranii group, owing mainly to injection-site side effects. Therefore, the power of the study may have been diminished by the high nonadherence rates with respect to the analysis of the primary outcome in the Mycobacterium indicus pranii group. Finally, an interaction may have occurred between adjunctive prednisolone and Mycobacterium indicus pranii, which could result in each one either increasing or reducing the effects of the other, because prednisolone is immunosuppressive and Mycobacterium indicus pranii is immunostimulatory [26].
8.1.6 Study conclusions
In conclusion, Mycobacterium indicus pranii administered for 3 months combined with adjunctive prednisolone therapy for 6 weeks did not have a positive effect on the combined outcome of death from any cause, constrictive pericarditis, or cardiac tamponade requiring pericardiocentesis. There was an increased risk of HIV-related cancer when both therapies were administered in HIV-positive patients. However, the incidence of hospitalization and pericardial constriction were reduced through the use of adjunctive glucocorticoids. In addition, the beneficial effects of prednisolone with respect to hospitalization and pericardial constriction were similar in HIV-negative and HIV-positive patients.