Chapter 19
Synapse Elimination
Overview
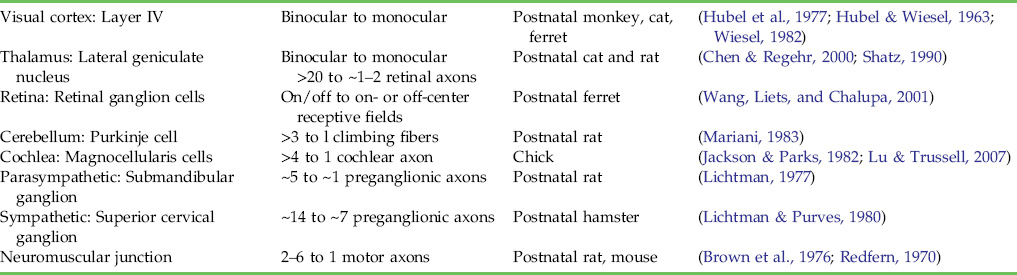
In the development of both central and peripheral nervous systems, synapse formation generates some connections that exist only transiently. Proof that synapses are eliminated comes from both anatomical and physiological studies showing that in many parts of the mammalian nervous system, presynaptic axons are connected to postsynaptic partners during development that they are not connected to at later stages. One unambiguous example of such loss is the change in innervation of mammalian neuromuscular junctions in early postnatal life. At birth, each neuromuscular junction receives convergent innervation from multiple motor axons (Fig. 19.1A and 19.1C). In adult muscles, however, only a single motor axon innervates each neuromuscular junction (Fig. 19.1B). Also in the sympathetic and parasympathetic autonomic ganglia of the peripheral nervous system, axons transiently supply extra inputs to each ganglion cell (Fig. 19.2). In the developing mammalian central nervous system (CNS), there are also several cases of documented synapse elimination (Table 19.1). For example, multiple climbing fibers innervate each cerebellar Purkinje cell at birth, whereas all but one is removed in early postnatal life. In the auditory system, developing neurons in the magnocellularis nucleus receive innervation from several inputs but in later life only one axonal input per neuron remains. Perhaps the best-known examples of synapse elimination occur in the visual system where some neurons that are initially activated by input from both eyes become monocularly driven later on.
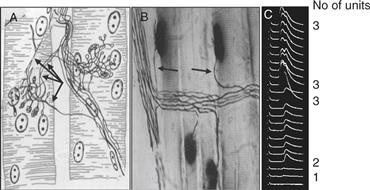
Figure 19.1 Evidence of synapse elimination at the neuromuscular junction. (A) Drawing of a silver stain made by J. Boeke in 1932 of the motor nerve terminals converging on two adjacent muscle fibers from the tongue muscle of a 4-day-old mouse. Each fiber is innervated by endings from several different axons (see arrows). (B) Photomicrograph of several adult muscle fibers from the rat extensor digitorum longus muscle. Each adult fiber receives innervation from a single axon (arrows). Axons are impregnated with silver and stained for cholinesterase (dark oval smudge on each muscle fiber). (C) Intracellular recordings from a muscle fiber in the neonatal rat diaphragm showing multiple innervation of a skeletal muscle fiber. Gradually increasing the strength of motor nerve stimulation elicits three different postsynaptic responses in this fiber. Similar assays in adult animals give only a single postsynaptic response that cannot be fractionated by graded stimulation. The preparation was partially curarized to prevent the endplate potentials from reaching threshold.
Figures adapted from (A) Boeke (1932); (B) Gorio, Marini, and Zanoni (1983); (C) Redfern (1970).
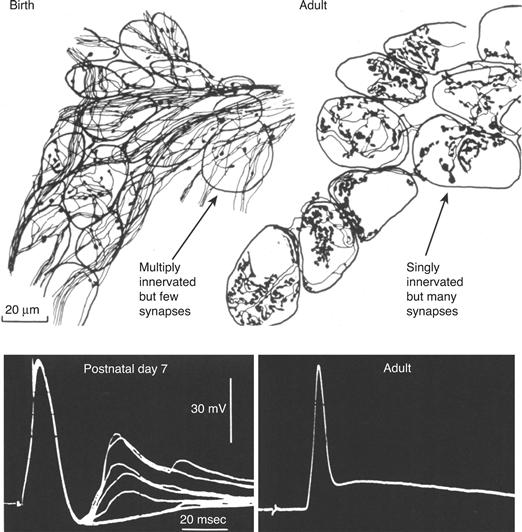
Figure 19.2 In the developing nervous system synapse number is increasing while axonal convergence is falling. The total number of preganglionic synapses on ganglion neurons in the rat submandibular ganglion increases in early postnatal life during the period of synapse elimination. Camera lucida drawings of clusters of ganglion neurons at birth and in adult animals that were treated with zinc iodide osmium. This reagent selectively fills terminal axons and synaptic boutons with a dense black precipitate. The number of synaptic boutons increases (compare the amount of synaptic boutons (arrows) at birth and on adult ganglion cells), and the number of preganglionic axons innervating each ganglion cell, identified by electrophysiological measurements, decreases (see the number of electrophysiological steps (arrow), each representing an input, at early postnatal life and in adult ganglion neurons, see lower panels). Thus axons that are not removed create new synapses to more than compensate for the loss of synapses from other axons.
From Lichtman (1977).
Table 19.1 Synapse Elimination in the Mammalian Nervous System
In all these cases, synapse elimination reduces the number of axons that innervate target cells, making the term input elimination perhaps a better descriptor. Input elimination, however, is only easy to measure in relatively unusual situations, such as when neurons end up with one axon or innervation from one eye. It remains unclear how common synapse elimination is in the more typical situation where neurons receive hundreds or even thousands of different axonal inputs from a diverse set of presynaptic cells. Thus in most parts of the developing nervous system, it remains unknown whether synapse elimination is occurring at all. The uncertainty is further confounded by contemporaneous synapse formation in the developing nervous system. As described following, some of the additional synapses are originating from axons that are already connected to a postsynaptic cell. Thus the total number of synapses impinging on a postsynaptic cell may even be increasing at the stage when there is a net reduction in the number of axonal inputs innervating the target cell. Thus assaying for synapse elimination requires quantifying a change in the number of innervating axons rather than counting the number of synapses, which in turn requires a means of counting axons, or at least having a situation where distinct but overlapping pathways can be separately labeled or stimulated (such as the two eyes). It seems likely that there is far more synapse elimination during development than presently documented. For example, stimulation of the optic nerve with progressively larger voltages to estimate the number of different retinal ganglion cells innervating individual lateral geniculate neurons has revealed a dramatic change from more than 20 innervating axons to only several during the first postnatal month (Chen & Regehr, 2000), a much larger change than had been previously suspected in that system.
Importantly, synapse elimination, despite causing a reduction in the number of sources of innervation to a target cell, does not lead to a net weakening of synaptic drive on target cells. In the example just mentioned, the retinogeniculate axons that persist undergo a greater than 50-fold increase in synaptic efficacy, which more than compensates for the loss of other axonal inputs to the same target cells. Analogous increases in synaptic efficacy of inputs that persist have been described for the excitatory drive to neurons of the magnocellularis nucleus (Lu & Trussell, 2007) and for the climbing fiber inputs to Purkinje cells in the cerebellum (Hashimoto & Kano, 2003). In each case, the inputs that remain increase their efficacy by 10-fold or more as other axons are eliminated. The same trend was seen many years ago in the parasympathetic submandibular ganglion, where the remaining single input was always far more powerful than any of the inputs at the earlier stage when five to six multiple axons converged to the same single target cell (Lichtman, 1977). Similarly, at the neuromuscular junction, the remaining motor axon increases its quantal content as other inputs are eliminated (Colman, Nabekura, & Lichtman, 1997). It thus appears that although some inputs are being removed, the survivors are potentiated. Such results raise the outstanding question of the degree to which the compensation is explained by changes in the efficacy of the maintained synapses or rather the elaboration of new synapses.
A partial answer to this question comes from studies in the peripheral submandibular ganglion. Here, the simplicity of the neuropil (most synapses are axosomatic) allows for direct counts of synapse number during the period of synapse elimination. In this ganglion, input elimination was accompanied by concurrent synapse addition by the remaining input. In particular, over the first postnatal month, there was approximately a twofold increase in the total number of synapses per ganglion cell, whereas the number of innervating axons was reduced fivefold (Fig. 19.2). This compensation indicates that the remaining axon on each ganglion cell must be increasing the number of synaptic contacts by approximately a factor of 10 as other axons lose all their connections to the same neuron. Such compensatory synaptogenesis means that assays of the total number of synapses at various developmental stages or following learning paradigms may belie a rather dramatic change in the source of the synapses.
It is also clear that in many situations the alterations occurring during this developmental reorganization are better described as a redistribution of synapses than as loss per se. The view is based on the fact that counts of the total number of neurons that innervate the entire target are not changing during the stage when the number of axons innervating each postsynaptic cell is decreasing (see, for example, Brown, Jansen, & Van Essen, 1976). Moreover, in several regions of the nervous system, it is known that synapse elimination occurs after the period of naturally occurring cell death is complete (see Chapter 18). Thus, as the elimination process reduces the number of axons converging on postsynaptic cells, it does not entirely remove axons from the postsynaptic target region but rather reduces the number of target cells innervated by each axon—that is, it reduces the axonal divergence (sometimes called fan-out) as individual axons restrict their synapses to a smaller number of postsynaptic target cells (Fig. 19.3). In muscle, axonal branch trimming during development has been long appreciated by an electrophysiological assay: the proportion of the total twitch tension an individual axon exerts decreases gradually over early postnatal life (Brown et al., 1976). This assay suggests that motor units (the term given to the number of muscle fibers innervated by a single motor axon) reduce their sizes by about threefold after birth.
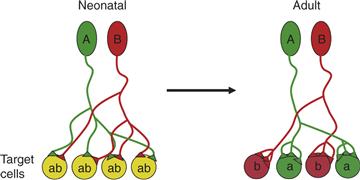
Figure 19.3 Changes in fan-in and fan-out at developing circuits. In neonatal vertebrates, neurons (A, green and B, red) project their axons to fan out to many target cells (each labeled ab). These nascent connections are typically weak and mediated only by a small number of synaptic contacts (triangles from the green and red axons). Axons undergo several structural rearrangements before reaching adulthood. First, axons disconnect from many target cells reducing their fan-out or divergence. Second, this rearrangement leads to less fan-in or convergence. Third, at the same time as axons disconnect from some target cells they are strengthening their connection with other target cells by increasing the number of synapses at their remaining targets.
Newer techniques of visualizing all the branches of single axons by transgenic expression of GFP (Feng et al., 2000) have, however, suggested that the changes are far more dramatic. Transgenic animals, in which only one axon is labeled, show that axonal arbors are about ten times larger at birth than in adults (Fig. 19.4A). These very large motor units imply that individual muscle fibers are innervated by ten or so axons because the total number of muscle fibers and neuromuscular junctions is fixed from birth onward. Direct evidence of this massive convergence on neuromuscular junctions was obtained by serial electron microscopy (Fig. 19.4B). Thus in muscle there is simultaneously extensive fan-in on neuromuscular junctions and fan-out of motor units in early life that is dramatically removed in early postnatal life.
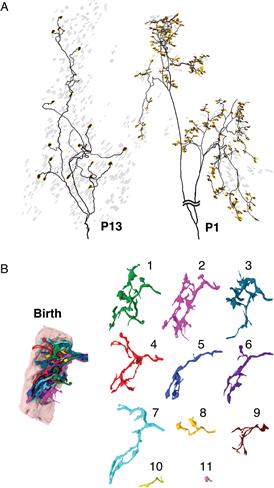
Figure 19.4 Profound changes in axonal arbors and synapses at birth. (A) Left, The axonal arbor of a motor axon’s motor unit from a postnatal day 13 mouse. The neuromuscular junctions innervated by this axon are highlighted (yellow), and the rest of the neuromuscular junctions are shown in gray. Right, The branching arbor of a motor unit in the same muscle at postnatal day 1. At this young age the axon arbor is dramatically more complicated, and it innervates manyfold more neuromuscular junctions (yellow). B, The image on the left shows a serial electron microscopy 3D reconstruction of a single neuromuscular junction at birth that was then traced to show that 11 different axons converged on the site. On the right, each of the 11 axons that innervate this neuromuscular junction at birth are separately displayed, showing that they are quite intermingled and none occupies a majority of the neuromuscular junction.
The transgenic expression of fluorescent proteins has allowed study of how this removal occurs. Direct observation of axonal branch retraction in living animals by time-lapse imaging shows that axons that are removed first become thin (axonal “atrophy”); they then disconnect from the postsynaptic site and temporarily possess a bulb-tipped free axonal ending called a retraction bulb (Fig. 19.5). The withdrawing axon sheds small spherical membrane-enclosed axonal fragments filled with synaptic organelles (axosomes) to be engulfed by surrounding Schwann cells (Fig. 19.6). The sites formerly occupied by the withdrawing axon are mostly taken over by the axon that remains at the junction (described in detail following).
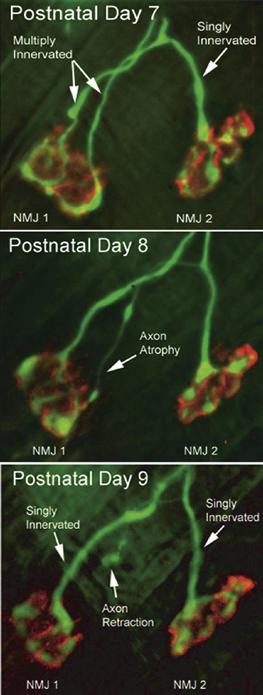
Figure 19.5 Time-lapse imaging shows how axonal branches are lost during synapse elimination at the neuromuscular junction. Two neuromuscular junctions (NMJ1 and NMJ2) were viewed in vivo on postnatal days 7, 8, and 9 in a transgenic mouse that expresses YFP in its motor axons (green). The acetylcholine receptors at the muscle fiber membrane are labeled with rhodamine-tagged α-bungarotoxin (red). On day 7, NMJ1 is multiply innervated, whereas NMJ2 is singly innervated by a branch of one of the axons that innervates NMJ1. One day later (postnatal day 8), one of the motor axons innervating NMJ1 becomes thinner all the way back to its branch point. When viewed on postnatal day 9, this thin branch is no longer connected to NMJ1 and now ends in a bulb-shaped swelling that is called a retraction bulb.
Modified from Keller-Peck et al. (2001).

Figure 19.6 Retracting axons shed material as they disappear. The inset shows a surface rendering of a serial electron microscopy reconstruction of a retraction bulb (green, arrowhead) during synapse elimination at a developing neuromuscular junction. The electron micrograph shows that the retracting axon contains clusters of vesicles and mitochondria. In the vicinity of the bulb but not connected are small spherical axonal fragments (axosomes, arrow) that are engulfed by glial cells (i.e., Schwann cells (SC), darker cytoplasm). Scale bars, 1 mm.
Summary
In the developing nervous system, both the number of axons that converge on postsynaptic cells and the number of postsynaptic cells an axon contacts decrease. The removal of inputs is due to a process of branch trimming. Concurrently, the remaining inputs appear to compensate by adding synaptic strength at least in part by the formation of new synapses. This redistribution refines synaptic circuitry by allowing an axon to strongly focus its innervation on a subset of the cells it initially contacted while each postsynaptic cell is restricted to responding to a subset of the axons that initially innervated it.
The Purpose of Synapse Elimination
At first sight, it might seem reasonable that synapse elimination is some form of error correction to rid the developing nervous system of outright connectivity mistakes. Indeed, there are clear examples of classes of nerve cell connections that disappear during development. For example, there are connections between mossy fiber axons and Purkinje cells in the developing cerebellum but not in the adult (Kalinovsky et al., 2011). In other situations, synapse elimination may sharpen specificity of the connections in a slightly more subtle way. For example, in some muscles there is a topographic map that matches the position of an axonal arbor to the location of the cell body within the motor neuron pool in the spinal cord. In these muscles synapse elimination makes these maps more precise (see, for example, Laskowski, Colman, Nelson, & Lichtman, 1998).
However, synapse elimination also occurs robustly in muscles and autonomic ganglia in which there is no evidence for intrinsic positional or other qualitative differences between axons that are maintained and those that are lost from any particular postsynaptic cell. Thus, while synapse elimination may be employed to refine the specificity of synaptic circuits, it seems to occur even in situations where it is not obvious that the synaptic connections that are maintained are from a different class of neuron than the ones that are eliminated.
An alternative hypothesis to the idea of error correction is that the loss of connections is a natural consequence of the extreme degree to which mammalian (and other vertebrate) nervous systems are composed of duplicated neurons (Lichtman & Colman, 2000). For example, pools of motor neurons that may number in the hundreds innervate individual skeletal muscles that contain thousands of muscle fibers. This reduplicated arrangement differs from the cellular plan in most parts of invertebrate nervous systems. For example, in the fly neuromuscular system, sometimes a single identified motor neuron innervates a muscle consisting of only one muscle fiber (Fig. 19.7A). Thus in mammals a cadre of nearly identical motor neurons that seem to have approximately equivalent roles project to a target consisting of a large population of roughly equivalent postsynaptic cells that constitute a muscle (Fig. 19.7B).
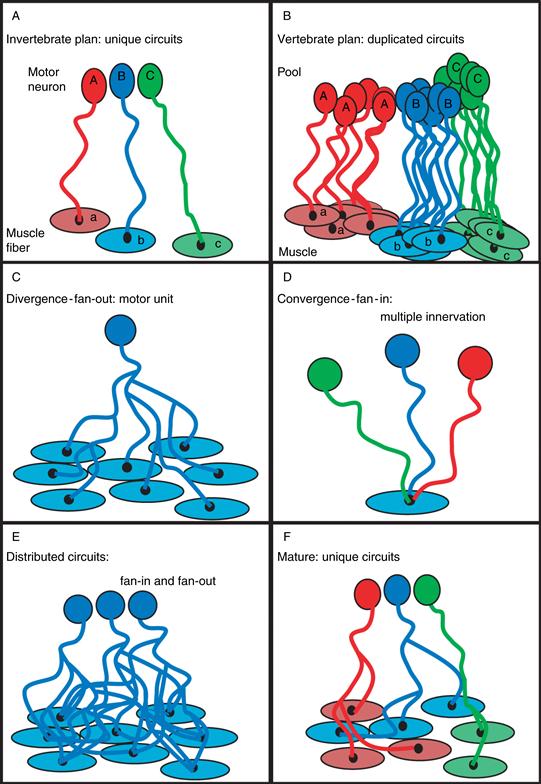
Figure 19.7 A diagram showing differences between the vertebrate and invertebrate synaptic circuits. (A) Invertebrates have small numbers (for example, sometimes just one motor neuron) of identifiable neurons innervating small numbers of identifiable target cells (e.g., sometimes just one muscle fiber), whereas in vertebrates, pools of similar neurons innervating targets contain hundreds or thousands of similar postsynaptic cells (B). The redundancy in the vertebrate nervous system allows a neuron to diverge (fan out) and innervate many equally appropriate target cells (in the neuromuscular system, this is called a motor unit) (C). The homogeneity of innervating neurons allows multiple axons to converge (fan in) on each target cell (D). As a result, vertebrate circuits contain a substantial amount of fan-in and fan-out (E), at least initially. Synapse elimination’s purpose may be to transform such a set of redundant circuits into multiple unique ones by trimming away the multiple innervation of target cells (F). The result of the widespread loss of synapses is the generation of thousands of nonredundant circuits from an initially much less specific innervation pattern.
There are two potential circuit consequences of such redundancy in pre- and postsynaptic populations. First, many postsynaptic neurons are appropriate matches for each presynaptic axon, causing substantial axonal divergence or fan-out (Fig. 19.7C). Second, many presynaptic neurons could be appropriate matches for each postsynaptic cell, causing substantial axonal convergence or fan-in (Fig. 19.7D). Therefore, given the redundancy in both pre- and postsynaptic populations, one might expect overlapping converging and diverging pathways (Fig. 19.7E). Indeed this is the pattern of innervation in the neuromuscular systems in mammals and many other vertebrates, but only during development.
Therefore, what is surprising is that this overlap is short-lived and circuits undergo a radical transformation in postnatal life. As already mentioned, during neuromuscular development, axonal branches are pruned in a highly selective way, causing the projection of each motor axon to become completely nonoverlapping and thus completely distinct from other axons’ projections (Fig. 19.7F). From a functional standpoint, once synapse elimination is complete, the recruitment of each motor axon gives rise to activation of a different set of muscle fibers and consequently a substantive increase in tension of the muscle. This stepwise tension increase is a necessary aspect of the size principle (see Chapter 18). Therefore, the circuit rearrangement has an important consequence on neuromuscular output—that is, behavior.
By parsing highly redundant circuitry into multiple, unique, functionally distinct circuits, synapse elimination may be an essential maturation step for synaptic circuits that begin with considerable redundancy, such as those in our own nervous systems and those of other terrestrial vertebrates. It is possible that this paring down strategy is less relevant in other kinds of animals. Indeed, in most invertebrates, identified neurons are the rule, and there is scant evidence for the type of neuronal redundancy seen throughout the mammalian nervous system. This difference suggests that a distinct neurodevelopmental strategy is at play in mammals and perhaps other vertebrate classes compared to the strategy used in the vast number of species in all the other subphyla. It is perhaps not a coincidence that another difference that partitions humans and other mammals from most invertebrates is that they seem highly dependent on experience for the acquisition of their behavioral repertoire. Invertebrate behavior is, to a greater degree, intrinsic and stereotyped. Compare, for example, the ease with which a newly hatched dragonfly takes wing versus the protracted period necessary for a human child to learn to walk. It is possible that the neuronal redundancy found in higher vertebrates that gives rise to overlapping convergent and divergent pathways is used in the acquisition of skills by the experience-mediated selection of connections.
This difference between mammals and invertebrates does not mean that synapses cannot be eliminated in invertebrates. Indeed, many studies have made the point that synaptic remodeling through addition and removal occurs in insect nervous systems (see, for example, Ding, Chao, Wang, & Shen, 2007; Eaton & Davis, 2003). The molecular mechanisms involved in invertebrate synaptic remodeling may provide insights into the mechanisms of synaptic loss and maintenance in higher vertebrate nervous systems. In worms, for example, the ubiquitin proteosome complex has been shown to regulate the elimination of motor neuron synapses onto vulval muscles (Ding et al., 2007). Similar degradation mechanisms may also be involved in synaptic remodeling of vertebrate neurons (Bingol et al., 2010; Li et al., 2010). The important point here, however, is that these mechanisms are used in mammals to alter the connectivity of the nervous system (i.e., to alter the number and identity of an axon’s postsynaptic targets). In most invertebrate systems, and perhaps lower vertebrates such as fish, these same mechanisms may play more of a role in modifying the strength of existing connections without altering the circuit’s wiring diagram.
Summary
In contrast to invertebrates, the nervous systems of terrestrial vertebrates contain reduplicated populations of neurons that serve each function. Elimination of synaptic connections during development may be an adaptation that converts highly overlapping connections of redundant neurons into unique circuits. Because this conversion may be based on experiences that affect the development of the nervous system, this process may tune the nervous systems of higher animals to the particular environment of each individual animal.
A Structural Analysis of Synapse Elimination at the Neuromuscular Junction
Thanks in large part to the power of fluorescence microscopy and the accessibility of the neuromuscular junction, it has been possible to describe the physical changes in axons and synapses that take place during synapse elimination. Interestingly, this purely descriptive analysis has yielded important clues and some mechanistic insights into the process of synapse elimination. As Yogi Berra said, “You can observe a lot by just watching.” Imaging neuromuscular junctions at various ages in early postnatal life makes clear the fact that the process of synapse elimination is not a sudden calamitous event but rather a protracted process. At birth, multiple axons converging at a neuromuscular junction are highly intermingled, and the extent of the receptor areas occupied by each is similar (Balice-Gordon, Chua, Nelson, & Lichtman 1993). These highly intermingled connections eventually become progressively segregated over several days (Figs. 19.4B and 19.8). The partitioning of synaptic areas associated with different axons suggests that there is a spatial component to the mechanism. One scenario, for example, is that each axon locally destabilizes other inputs in their immediate vicinity but cannot as efficiently affect slightly more distant branches of the same axons. If the destabilization of an input was followed in turn by the takeover of its synaptic sites by the remaining axon, then an axon with a larger consolidated area might begin to destabilize more distant inputs. The idea of progressive consolidation was put to the test by the use of transgenic mice expressing different-color fluorescent proteins in individual axons. With these animals it is possible to obtain a precise map of the territories occupied by two axons at the same neuromuscular junction. In vivo time-lapse imaging of a multiple innervated neuromuscular junction over several days showed both gradual withdrawal of one axon from postsynaptic sites and a corresponding expansion of another axon to take over those synaptic sites (Fig. 19.9). This result may mean that axons are vying to occupy the same sites. Interestingly, it was not inevitable that the withdrawal of one axon was followed by the takeover of its sites by another input. In some cases, an axon that was already somewhat segregated from the other input would vacate its postsynaptic territory, but rather than being replaced by the remaining axon, its acetylcholine receptor-rich postsynaptic site would disappear. Synaptic loss without reoccupation by another input suggested that the takeover process per se was not causing withdrawal of the other axon. These observations suggest rather that synaptic takeover might be a response to the recent synaptic vacancy.
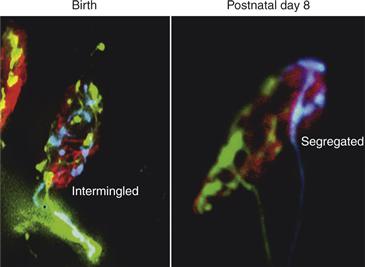
Figure 19.8 Segregation of synaptic territory as axons compete during synapse elimination. Double transgenic mice expressing YFP and CFP in different motor axons were used to observe axons’ nerve terminal interactions. At birth is shown two competing nerve terminals, one expressing CFP (blue) and the other YFP (green) at this neuromuscular junction, which are intermingled. By the second postnatal week, however, YFP and CFP axon terminals are generally completely segregated from each other in multiply innervated junctions. Red is fluorescently tagged α-bungarotoxin that binds to AChRs.
Modified from Gan and Lichtman (1998).
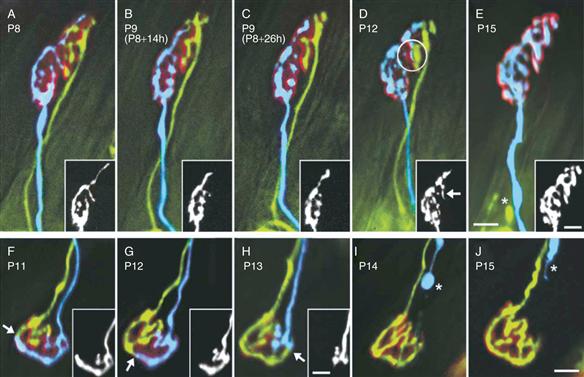
Figure 19.9 In vivo imaging shows takeover of synaptic territory by the remaining axon during the period of synapse elimination. Neuromuscular junctions in transgenic mice that express YFP (yellow) and CFP (blue) were imaged multiple times during early postnatal life. (A–E) views of one neuromuscular junction between P8 and P15. The CFP axon takes over occupancy of the postsynaptic sites (labeled red) in the upper parts of the junction that were formerly innervated by the YFP axon. The YFP labeled axon withdrew until only a retraction bulb remained (E, asterisk). At P12, a process of the CFP axon had begun to invade the territory of the YFP axon (D, circle and arrow in inset). (F–J) Although the CFP axon (blue and insets) has greater terminal area (~70%) at the first view, it progressively withdraws from the junction (arrows). Its retraction bulb can be seen in (I) and (J) (asterisks). Scale bars = 10 μm. Insets show the blue axons.
These time-lapse studies also demonstrated that the shift in favor of one axon was not irreversible. In some junctions, for example, it was hard to predict which axon might ultimately be maintained because the input with the majority of the territory shifted back and forth. Such flips in which axon seemed to be dominant suggest that the outcome is not preordained. Instead, axons at multiply innervated neuromuscular junctions appear to be in the midst of a highly dynamic interaction to determine which axon is most likely to stay.
Insights into the regulation of this dynamism come from analyses of each of the branches of individual motor axons. In lines of transgenic mice in which fluorescent proteins are expressed in a very small subset of motor neurons, it has been possible to examine all the branches of one axon during the developmental period when branches are being pruned. These studies show that axonal branch loss is occurring asynchronously among all the branches of one axon (Fig. 19.10). Thus, while some branches are recently eliminated (i.e., retraction bulbs), other branches are still connected to neuromuscular junctions that are multiply innervated. This range suggests that the fate of each branch is controlled independently. If axonal branches of one neuron are interacting with different axons at each of its neuromuscular junctions, then perhaps the rate of synapse elimination is regulated by which particular axons are co-innervating each of these junctions. These interactions might not only determine the rate but also the fates of the terminals—that is, which neuron’s branches are maintained and which are eliminated. For example, an axon might be eliminated quickly at neuromuscular junctions innervated by some other neurons but fare better at junctions innervated by other axons.
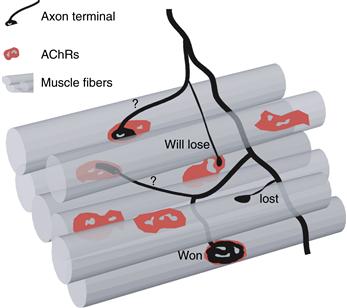
Figure 19.10 Asynchronous synapse elimination among the branches of one axon. Using transgenic animals that express YFP in a small subset of motor axons, it was possible to monitor the behavior of multiple branches of the same axon. This diagram shows the typical result for an axon in the midst of the synapse elimination process. Represented in red are the AChRs on each muscle fiber (represented as gray tubes). This motor axon (black) has won the competition on the bottom muscle fiber and occupies the entire receptor plaque. The same axon has lost the competition for the adjacent muscle fiber, where only a retraction bulb remains. The three other neuromuscular junctions that this axon innervates are still undergoing competition. In one case a small portion of AChRs is being innervated by this axon. It is likely that this synapse will be eliminated. On the other two muscle fibers, each axon terminal occupies 50% of the AChRs. This indicates that its fate is not yet determined in these junctions.
Adapted from Lichtman and Colman (2000).
To explore this possibility, transgenic mice with only two labeled axons (one yellow and one cyan) were generated to examine each of the neuromuscular junctions co-occupied by the same two axons within a developing muscle. The result was dramatic: at each of the shared neuromuscular junctions, the two axons often seemed to be in the same relative state (Fig. 19.11). Thus if the cyan axon was occupying only a small amount of territory at one junction that it shared with the yellow axon, then it occupied a small amount of territory at all or nearly all the other junctions it shared with the same yellow axon. However, where the cyan axon was interacting with other axons, its fate could be quite different. This result argued that the fate of axonal branches and the rate at which they are eliminated are related to the identity of the co-innervating axons. But why might the yellow axon be consistently “better” than the blue at all the co-innervated neuromuscular junctions they share? Counts of the total number of neuromuscular junctions each axon innervated (i.e., its motor unit size) provided a hint; the outcome of synapse elimination seemed to depend on the relative sizes of the two axons’ motor units. In particular, neurons with larger motor units (such as the cyan axon in Fig. 19.11) were at a disadvantage when confronting neurons with smaller arborizations (such as the yellow axon in Fig. 19.11). One interpretation of this result is that axons with few branches in a muscle could dedicate more resources to a multiply innervated neuromuscular junction than neurons with a larger number of branches that are in some sense overextended. If this idea is correct, then the consequence of losing branches for an axon (such as the cyan one in Fig. 19.11) is that it can now dedicate more resources to its remaining multiply innervated junctions shared with other axons.
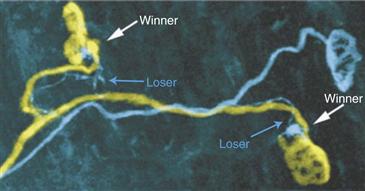
Figure 19.11 Axonal fate at individual neuromuscular junctions is related to the identity of the competing axons. Using transgenic animals that express YFP (yellow) and CFP (blue) in a small subset of motor axons, it was possible to reconstruct two motor units in which several neuromuscular junctions are innervated by the exact same two axons, one expressing YFP and the other CFP. At these coinnervated junctions, it appears that the same outcome is occurring at each. In this case, the YFP axon always occupied more territory than the CFP axon. Note also that the axon calibers of the YFP axon branches are thicker than CFP branches to the same junctions. Thus synapse elimination seems to be biased such that when the same two axons compete, the fate is the same at each coinnervated neuromuscular junction.
Image from Kasthuri and Lichtman (2003).
These results raise the obvious question of what resources might be in limited supply in an axon that could affect synapse maintenance. One popular idea is that the synaptic activity of an axon terminal is a key determinant in its ultimate fate. Is it possible that large motor units have fewer synaptic resources (e.g., synaptic vesicles per synapse) than small motor units? This idea was tested by generating mice in which a subset of axons had diminished choline acetyltransferase (ChAT, the enzyme that synthesizes the acetylcholine; Buffelli et al., 2003). The results showed that when axons containing normal levels of ChAT co-innervated junctions with axons that had subnormal amounts, the subnormal axons typically occupied smaller territories. This result is consistent with the idea that axons that have the greatest number of synaptic branches may be at a disadvantage because they cannot maintain sufficient resources to drive postsynaptic cells as well as axons with fewer branches.
Summary
Structural studies of synapse elimination at the neuromuscular junction have provided a number of clues as to how and why branches are pruned. One important insight is that synapse elimination at individual neuromuscular junctions may be part of a larger-scale circuit optimization. This optimization may be occurring to ensure that all the connections that are maintained into adulthood are sufficiently strong that they can consistently drive the postsynaptic cell to threshold. Perhaps such an optimization requires axons to forfeit some of their synaptic connections to ensure that the ones that remain have sufficient resources to be consistently efficacious.
A Role for Interaxonal Competition and Activity
A number of lines of evidence suggest that the loss of synapses is the consequence of competition. The word competition, however, has many different meanings, and which of these definitions is most relevant to synaptic development is an area of some debate (Lichtman & Colman, 2000; Ribchester, 1992; Ribchester & Barry, 1994). In broad terms, competition occurs when more than one individual (in this case, more than one axon) is capable of having the same fate (e.g., sole occupation of a neuromuscular junction), and the probability of having that fate is related inversely to the number of such individuals. The point of defining competition so broadly is to emphasize that competition does not imply what kind of mechanism drives the outcome; even lotteries, where winners are picked by random, are competitions. Synapse elimination occurring on muscle fiber cells or cerebellar Purkinje cells is thought to be competitive because there is always only one axon remaining at the completion of the process, and therefore more than one axon cannot share the same fate.
Importantly, which particular axon is maintained does not seem to be preordained. There is no evidence of an extensive molecular specificity that uniquely matches each motor axon to an exclusive subset of muscle fibers or each climbing fiber to a matching set of Purkinje cells. Moreover, if there were some molecular blueprint, one might expect some stereotypy in that pattern between the same muscle in different individuals. However, painstaking axon tracing of all the motor units in a single muscle has revealed a complete lack of similarity in the organization of axons in many instantiations of the identical muscle (including in the left and right side of the same animal), arguing that no molecular patterning is establishing the particular wiring diagram. But if it is a competition that explains the variable outcomes, how is this competition being driven? Competitive mechanisms range from situations where the contestants interact with each other directly (e.g., a sumo wrestling match) to mechanisms where the contestants have little or nothing to do with each other, and a third party (a judge) decides the outcome (e.g., competition for a Pulitzer Prize). Evidence in the neuromuscular system suggests that synaptic competition may be more akin to the latter alternative, with the muscle fiber playing the role of judge.
Neuromuscular System
In 1970, Paul Redfern (1970) published the first physiological report of synapse elimination. He found that rat diaphragm muscle fibers were innervated by several motor neurons in the first postnatal week, but this multiple innervation was short lived; by two weeks of age, all muscle fibers were singly innervated (Fig. 19.1). At the time, he suggested that the extra innervation may be explained by the presence of motor neurons that project to a muscle in early life but subsequently undergo cell death. A few years later, this idea was shown to be incorrect when the tension elicited by activating single motor axons was shown to drop precipitously over the same period of early postnatal life, indicating that synapse elimination was accompanied by motor unit branch trimming rather than wholesale motor neuron loss (an event that occurs in the prenatal period) (Brown et al., 1976). Brown and colleagues also made the important point that because all neuromuscular junctions ended up with exactly one innervating axon, the elimination process was likely explained by interaxonal competition—otherwise all axons should leave a neuromuscular junction, or more than one converging axon should persist into adulthood. These two seminal reports stimulated many investigators to seek an understanding of the underlying mechanisms. Much of this effort has focused on the role of activity in synapse elimination. Many lines of evidence show that modifying neuromuscular activity has a large effect on synapse elimination (Thompson, 1985). However, it has remained unclear how activity might mediate interaxonal competition, as conflicting evidence suggested that either active (Ridge & Betz, 1984) or, surprisingly, inactive axons (Callaway, Soha, & Van Essen, 1987) are at an advantage. At first glance, it may seem improbable that inactive axons could outcompete active ones (a result that is opposite to one discussed later, on the effects of monocular deprivation in visual system synapse elimination). Callaway et al. (1987) argued that the way muscles are used, the axons that are recruited most infrequently were the ones that in adults had the largest motor units. If their large sizes resulted from less branch trimming during development, then inactive axons might indeed have the advantage in synaptic competitions. Further complicating matters is the fact that the total number of axons innervating the muscle does not appear to change (Brown et al., 1976). This constancy implies that no individual axon’s activity pattern could be considered the worst, because all axons maintain some neuromuscular junctions and thus all axons must outcompete other axons at some junctions.
Other experiments have questioned whether activity is even necessary for synapse elimination. For example, after reinnervation of adult muscle, evidence has been obtained showing that electrically silent inputs can displace other electrically silent inputs (Costanzo, Barry, & Ribchester, 2000). Moreover, in some cases, the presence of activity does not invariably lead to neuromuscular synapse elimination (Costanzo, Barry, & Ribchester, 1999).
As these conflicting experimental results demonstrate, the role of activity in synapse elimination is not easily summarized. One way to obtain a clearer idea of the role of activity is to view “activity” not as one phenomenon but as several different influences—for example:
• Synaptic competition may be affected by the relative efficacy (i.e., amplitude of the postsynaptic potential) of different axons attempting to drive the postsynaptic cell to threshold, with the most powerful input being favored.
• Alternatively, the firing frequency of an axon (i.e., action potentials per second) may have a negative impact, especially if the axon has a large arbor and thus insufficient resources to maintain efficacious synaptic transmission throughout its terminal branches.
• Finally, activity may affect the electrical properties of the postsynaptic cell or cell region, either encouraging synapse elimination (via postsynaptic activity) or preventing it by encouraging synapse formation (via the absence of postsynaptic activity) (Fig. 19.12).
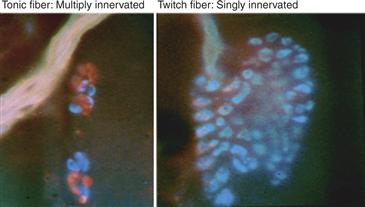
Figure 19.12 Postsynaptic cells that don’t fire action potentials don’t undergo synapse elimination. Photomicrographs of multiply and singly innervated snake muscle fibers. The singly innervated neuromuscular junctions are on twitch muscle fibers that have voltage-sensitive sodium channels. The multiply innervated neuromuscular junctions are found on tonic muscle fibers that do not have regenerative potentials. Labeling of different axons with different colors was accomplished by activity-dependent fluorescent labeling of axon terminals.
Perhaps the most attractive hypothesis is that difference in activity between axons is critical for synapse elimination to occur. Indeed, because an axon has many synaptic release sites impinging on one postsynaptic cell, which do not seem to compete with themselves, interaxonal activity differences may be a crucial element of the competition. An experiment in which one part of a neuromuscular junction was desynchronized from the rest by silencing its acetylcholine receptors with alpha bungarotoxin (Box 19.1) examined how activity differences might give rise to synapse elimination. Focal postsynaptic silencing at one site within an otherwise normally active adult neuromuscular junction induced synapse withdrawal from the silenced site (Fig. 19.13). Postsynaptic silencing of an entire neuromuscular junction, however, did not cause synapse loss. These results suggested that synaptic activity at some synaptic sites can induce synaptic destabilization of other sites if they are not active at the same time. This view of the way activity modifies synaptic connections is related to the well-known theory of plasticity: Hebb’s postulate (Hebb, 1949; see Chapter 52). Although Hebb argued that inputs that are consistently active when the postsynaptic cell is active are strengthened, this idea is the logical obverse: synapses that consistently fail to excite the postsynaptic cell when the cell is being activated are eliminated (Lichtman & Balice-Gordon, 1990; Stent, 1973). How might active synaptic sites destabilize silent ones? One suggestion is that active synapses generate two kinds of postsynaptic signals: one that protects them from the destabilizing effects of activity and one that punishes other inputs that are not active at the same time (Fig. 19.14). The physical basis of these protective and punishment signals remains unclear. Since neurotransmitter receptors are sometimes permeable to calcium, and the activity-induced depolarization can also raise intracellular calcium levels, one idea is that calcium signaling serves one or both of these roles.
Box 19.1 α-Bungarotoxin
A number of different dyes, stains, and markers are useful in revealing synaptic structure and function. Some of the more powerful have been borrowed from nature. Many toxins and poisons bind to specific proteins. One example is the snake toxin a-bungarotoxin (α-btx). This toxin is a constituent of the venom of a Krait snake of the species bungarus. The lethality of α-btx is the consequence of its ability to bind to the α subunits of nicotinic AChRs in skeletal muscle cells of vertebrates (with a few notable exceptions: snakes, mongooses, and hedgehogs). Because α-btx is an irreversible competitive antagonist of the AChR, its binding thus paralyzes and suffocates the prey. Researchers have taken advantage of this snake toxin to study many aspects of the AChR. For example, α-btx was used to purify AChRs from Torpedo membranes. Furthermore, the toxin can be conjugated to radioactive markers or to fluorescent molecules that can be seen in the microscope to stain receptors on muscle cells to determine their distribution, stability, and motility in the membrane. Because the toxin binds essentially irreversibly to the receptor in the muscle fiber membrane, receptors can be labeled once and then their behavior followed over time. This approach has provided substantial evidence about the stability of synaptic regions on muscle fibers, the lifetime of receptors in the membrane at the junctional sites, and how AChRs move within the plane of the membrane. For studies of synapse elimination in particular, the toxin also has been used to selectively inactivate some regions of a synapse by “puffing” it locally over a small region of a junction.
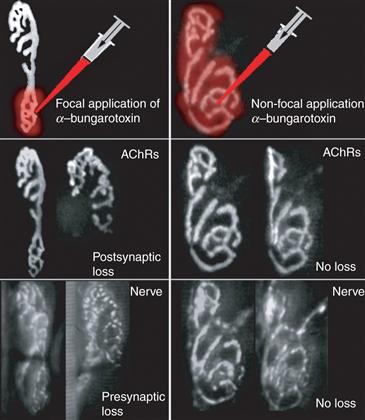
Figure 19.13 An experimental test of the role of postsynaptic activity in synapse elimination. If axons converging on the same postsynaptic cell were competing based on their efficacy in activating the postsynaptic cell, then locally blocking synaptic transmission at one axon’s synaptic site should lead to its elimination and the maintenance of the other unblocked inputs. In this test, focal blockade of neuromuscular transmission was accomplished by applying saturating doses of α-bungarotoxin with a fine glass pipette (upper panels, red) to a neuromuscular junction in a living mouse. The AChR sites (middle panels) and the nerve terminals (lower panels) were viewed at the time of blockade and several times over the next few weeks. Following focal blockade (left panels), progressive loss of the nerve terminal staining and AChRs (arrows) was observed in regions previously saturated with α-bungarotoxin. However, when the entire neuromuscular junction was blocked (right panels), there was no loss. These results argue that active postsynaptic synaptic sites can cause the disassembly of inactive sites.
Adapted from Balice-Gordon and Lichtman (1994).
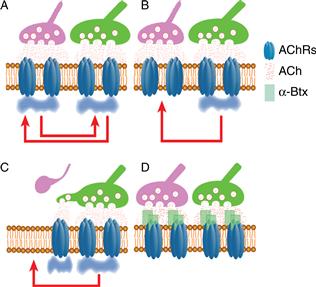
Figure 19.14 Putative mechanism for a postsynaptic role in synapse elimination. Postsynaptic receptor activation may elicit two opposing signals within a muscle fiber. One consequence of receptor activation is a “punishment” signal (here designated as red arrows) that causes destabilization of synaptic sites. Receptor activation may also generate a “protective” signal (here designated as blue clouds) that locally prohibits the punishment signals from destabilizing synapses in the vicinity of where receptor activation recently occurred. (A) When all the receptors are activated synchronously, as might occur before birth when motor neurons are electrically coupled (Personius & Balice-Gordon, 2001), there is no synaptic destabilization (due to protective blue clouds everywhere). (B) However, later in development, when two inputs are activated asynchronously, the active synaptic sites at any time point are protected (i.e., the synaptic sites beneath the active green axon are protected by a local blue cloud), whereas the asynchronously activated synapses (i.e., the synaptic sites under the pink axon) are not protected. (C) This asynchrony allows the more powerful input to destabilize the weaker one. This destabilization can lead to nerve and postsynaptic disappearance and/or nerve withdrawal, followed quickly by takeover of its former synaptic sites by the remaining axon, which would restabilize the site. (D) If all synaptic sites are inactive, as might occur with α-bungarotoxin application that inactivates nicotinic AChRs, then there are no blue clouds to protect synaptic sites, but also no red arrows to destabilize them. Thus synaptically silent and synchronously active synaptic sites (see panel A) do not undergo synapse elimination.
Idea adapted from Jennings (1994).
This view of the role of activity implies that if all axons were firing synchronously, then synapse elimination would not occur, which is exactly the conclusion reached using a regeneration model for synapse elimination (Busetto, Buffelli, Tognana, Bellico, & Cangiano, 2000). Interestingly, during the period of naturally occurring synapse elimination, there appears to be a switch from synchronous to asynchronous activity patterns (Buffelli, Busetto, Cangiano, & Cangiano, 2002; Personius & Balice-Gordon, 2001). It has been suggested that a gradual loss of electrical coupling among motor neurons may be the reason synapse elimination begins. This hypothesis was tested in mice that lack a gap junction protein (connexin 40) in which motor neuronal electrical coupling is reduced. In Cx40−/− muscles, synapse elimination was significantly accelerated, suggesting that asynchronous firing of neurons enhances synapse elimination (Personius, Chang, Mentis, O’Donovan, & Balice-Gordon, 2007).
Such an activity-based mechanism for synapse elimination would tend to pit the synchronously active synaptic terminals of one axon on a given postsynaptic cell (a synaptic “cartel”) against the terminals of other axons contacting the same target cell. In this way an axon’s terminals on one postsynaptic cell cannot compete against themselves but rather serve as a competitive unit vying against the cartels of other axons.
Visual Cortex
Classic studies on the visual system by Hubel and Wiesel were the first to suggest that competition in fact was driving synaptic reorganization in the developing brain (Hubel & Wiesel, 1963; Hubel, Wiesel, & LeVay, 1977). In most species of young mammals, input neurons to layer IV in the visual cortex can be activated by inputs driven from both the left eye and the right eye—that is, they are driven binocularly. Subsequently, however, in many species, cortical input neurons become strongly dominated by either the right or left eye, but not both (Fig. 19.15). In agreement with this physiological result, the terminal arbors of the geniculocortical axons from the two eyes overlap in early postnatal life (Hubel et al., 1977; but see Horton & Hocking, 1996). However, with time, their projections appear to resolve into a striking pattern of alternating stripes known as ocular dominance columns (Fig. 19.15). This anatomical result was based on anterograde transneuronal transport of radioactive amino acid that was injected into one eye and passed through the thalamus to label the eye-specific axons in the optic radiation (Hubel et al., 1977). Despite the remarkable clarity of these stripes, their functional significance is not well understood, and in some mammals, such as rodents and new world monkeys, ocular dominance columns are absent (Horton & Adams, 2005; Livingstone, 1996).
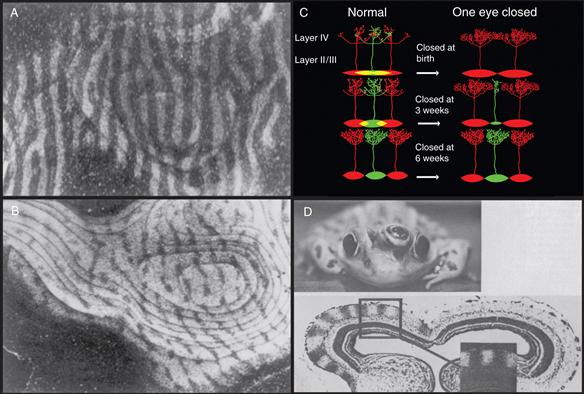
Figure 19.15 Synapse elimination in the visual system. (A) Ocular dominance columns of the neonatal monkey primary visual cortex in layer IVC, revealed by injecting [3H]-proline into the vitreous of one eye. Light stripes (columns) represent sites containing the anterograde transported 3H-amino acid from the injected eye. Dark regions are occupied by axons driven by the other eye. (B) Monocular deprivation by lid suture of one eye (2 weeks after birth for a period of 18 months) resulted in the shrinkage of the columns representing the deprived eye (dark stripes) and an expansion of the columns of the nondeprived eye (light stripes). (C) A schematic representation of ocular dominance column development represents the way in which a gradual segregation ocular dominance column could lead to the end of the critical period and progressively more modest effects of monocular deprivation as development ensues. (Top) At birth the afferents from the two eyes (red and green ovals) overlap completely in layer IV, and thus each eye is capable of maintaining inputs everywhere. At this young age, monocular deprivation would allow the nondeprived eye to remain in all parts of layer IV so that the entire cortex would be dominated by the red inputs. (Middle) In nondeprived animals, the two sets of afferents become progressively more segregated with age, meaning that by three weeks there would be regions of layer IV that are exclusively driven by the red or, as shown, green afferents. Once an eye’s inputs are removed from a territory, it can no longer reoccupy that territory when the other eye is silenced. Hence monocular deprivation (that begins at three weeks) will spare a small strip of the inactive eye’s territory (in this case the green regions). (Bottom) Once segregation is complete then monocular deprivation has no effect and the critical period is over. (D) A remarkable example showing how interactions between two eyes can cause segregation was found in frogs in which a third eye is implanted (at the tadpole stage) next to one of the eyes and projects with the native eye to the same optic tectum (ordinarily each frog tectum is monocular). After injection of H3-proline into the normal eye, one optic tectum of a three-eyed frog shows dark and light bands strikingly similar to the ocular dominance columns observed in monkeys (D).
A–C adapted from Hubel et al. (1977); D from Constantine-Paton and Law (1978).
During development, the ocular dominance columns’ organization is less obvious because there is overlap in the thalamic inputs driven by the right and left eye to layer IV. Anatomically as development proceeds, each eye’s columns become narrower and eventually almost nonoverlapping (especially in primates). Even though the narrowing in the widths of right and left eye ocular dominance columns is likely due to the loss of axonal branches in overlapping regions (Antonini & Stryker, 1993), this retraction of branches should not be taken to mean that the total number of thalamo-cortical synapses in layer IV is decreasing during this period. Similar to the peripheral nervous system, the axons associated with each eye elaborate many new synapses that more than compensate numerically for the lost connections of the withdrawing axons (Crowley & Katz, 2000; Erisir & Dreusicke, 2005). In other words, the process of ocular dominance column formation is one in which individual arbors lose synaptic connections with some targets but gain connections with others. Thus elimination restricts the neuronal population that is directly driven by each eye, but the synaptic addition strengthens the influence of one eye on the regions of cortex it continues to drive.
The most interesting aspect of this segregation is that the gradual removal of overlap in the two eyes’ input streams leading to equally sized ocular dominance columns is not inevitable. Hubel and Wiesel showed that during a developmental critical period in early postnatal life, the widths of these columns can be dramatically and permanently changed by alterations in the relative amounts of visual experience in the two eyes. In particular, the outcome of the segregation can be radically skewed in favor of one eye if the activity of the other eye is decreased (e.g., by patching one eye). This monocular deprivation results in larger columns for the open eye and smaller columns for the deprived eye (Fig. 19.15). Once the critical period of sensitivity is passed (approximately the seventh postnatal week in kittens, the tenth week in ferrets, and the twentieth week in rhesus monkeys), the widths of the ocular dominance columns are fixed and no longer subject to shifts based on visual experience. Remarkably, even long-term monocular deprivation (of decades or more) apparently has little effect on the width of ocular dominance columns if the visual deprivation is begun after the critical period is over (approximately six years in humans; Keech & Kutschke, 1995). For example, in human patients in which one eye was removed for surgical reasons in adulthood or late childhood, postmortem analysis of the visual cortex indicated that eye removal has little or no effect on the width of the ocular dominance columns dominated by the removed eye (Horton & Hocking, 1998). What might account for this dramatic change in sensitivity?
One idea is that during the critical period, thalamic afferents driven by the two eyes compete for control of the cortical neurons that they share temporarily. If each eye has the same average amount of activity, each ended up with similar amounts of cortical territory. However, if there were imbalances between the eyes in terms of visual experience, the outcome tipped the segregation in favor of one eye over the other. The skewing that resulted from depriving one eye of vision was due both to additional losses in the connections driven by the inactive eye (shrinking its ocular dominance columns) and to additional maintenance of the connections from the normally active nondeprived eye (maintaining its columns at the wider width it had at an earlier age) (Fig. 19.15). Ordinarily, each eye’s afferents would relinquish its connections with approximately half of its postsynaptic target cells in visual cortex. Furthermore, binocular eye closure during the critical period appears to have far less serious effects than monocular occlusion. These results support the idea that synapse elimination is due to an activity-mediated competitive interaction between the connections driven by the two eyes. Because binocular deprivation has less dramatic effects on ocular dominance columns than monocular deprivation, here, as at the neuromuscular junction, active synaptic inputs seem to play a role in destabilizing inactive inputs.
Though these conclusions appear straightforward, the roles of activity in cortical refinements may need reevaluation as new investigations show that the mechanisms may be more complicated than originally imagined. In studies of the rodent visual system, evidence suggests roles for both activity-dependent and -independent factors in developmental axonal refinements. Although mice lack ocular dominance columns, they do have a binocular cortical region that becomes progressively smaller as development proceeds. This shrinkage can be shifted with monocular deprivation during a critical period (Antonini, Fagiolini, & Stryker, 1999). This system has been used to show an important role of inhibitory circuits in both the establishment and maintenance of the critical period (Hensch, 2005). For example, deletion of the gene for glutamic acid decarboxylase, the enzyme that is responsible for the synthesis of the inhibitory neurotransmitter, GABA, prevents visual cortical refinements (Fagiolini & Hensch, 2000). Interestingly, these refinements can be reinitiated at any age by injecting GABA receptor agonists such as benzodiazepines into visual cortex. Thus intracortical inhibitory circuitry may be sufficient to trigger the opening or closure of the critical period in mice (Hensch, 2004; Hensch et al., 1998).
In mouse visual cortex, it has also been possible to study the sharpening of the retinotopic map in early postnatal life. The small receptive fields seen in adult visual cortex emerge from shrinkage of receptive field size in development (Issa, Trachtenberg, Chapman, Zahs, & Stryker, 1999). Activity plays a complex role in this sharpening; the effects of deprivation of formed vision (by lid suture) are different than the effects of pharmacological blockade or enucleation (Smith & Trachtenberg, 2007). A contralateral eye that is sensing light through a sutured lid impedes the refinement of the open eye’s central projection, whereas either an open, or completely silent, contralateral eye does not. This result suggests that when inputs are synchronous (i.e., both eyes open), the activity-mediated refinements that shrink receptive fields occur more efficiently than when the same cortical neurons are receiving inputs with different activity patterns (i.e., in an animal with one open and one sutured eye). On the other hand, when one eye is entirely silent (i.e., by enucleation or pharmacological blockade), then the refinement of the open eye’s inputs can still occur because in this case, there is no competing activity pattern from another eye. Thus some kinds of refinements may be mediated by cooperative interactions between the two eyes rather than competitive ones. One recent trend is that experiments that might have previously been interpreted strictly in terms of activity-mediated competition between different axons (à la Hubel and Wiesel) are now recast in terms of homosynaptic mechanisms of potentiation and depression or mechanisms of synaptic homeostasis (see Chapter 52). These newer frameworks for thinking about critical periods suggest that many different regulatory mechanisms working simultaneously may help to ensure that the right numbers and kinds of synapses survive the period of developmental refinements.
Thalamus
Separation of the inputs from the two eyes occurs twice in the visual system. Prior to the emergence of cortical ocular dominance columns, eye input to the lateral geniculate nucleus of the thalamus segregates into layers rather than columns. In embryonic cats, axon terminals of ganglion cells from the two eyes overlap extensively within the lateral geniculate nucleus before gradually segregating to form the characteristic eye-specific layers by birth. As in the cortex, this refinement process involves both the retraction of axonal branches from inappropriate regions of the geniculate nucleus and the elaboration of processes within the correct eye layer (Shatz, 1990). Physiological studies support anatomical observations that geniculate neurons initially are driven binocularly but maintain the axonal input from only one eye at maturity (Shatz, 1990). There is also a dramatic change in the convergence of retinal ganglion cell input to thalamic neurons related to a shrinkage in receptive field size (Chen & Regehr, 2000; Tavazoie & Reid, 2000) (Fig. 19.16).
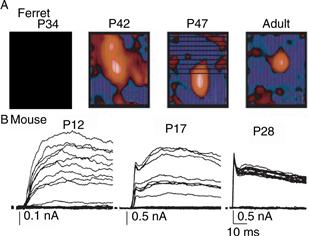
Figure 19.16 Synapse elimination in the lateral geniculate nucleus of the thalamus. (A) In the ferret lateral geniculate nucleus, receptive fields of geniculate neurons are larger and much more diffuse at one month of age than those receptive fields observed in adult neurons. Red regions represent the receptive field map of geniculate neurons that correspond to areas excited by bright stimuli. Note that red areas become smaller as development proceeds. The shrinkage in the receptive field is likely to result from the elimination of the convergence of multiple retinal afferents onto each geniculate neuron (B). At P12 in mouse, multiple retinogeniculate axons are recruited as stimulation intensities to the bundle of axons is increased (see also Fig. 19.1C). At P17 there are fewer steps, and after P28, only one or two inputs innervate each geniculate neuron (i.e., no steps in the evoked-synaptic currents are seen even though optic nerve stimulation is increased).
(A) Adapted from Tavazoie and Reid (2000). (B) Adapted from Chen and Regehr (2000).
It is likely that spontaneous activity as opposed to actual visual experience is important in the segregation of retinogeniculate connections in the thalamus. This view is based on the fact that in cats, ferrets, and monkeys, the eye-specific layers are established well before the retina is sensitive to light and is dependent on the spontaneous activity of these inputs. What information may be contained in the spontaneous activity patterns of immature retinas that could lead to eye-specific lamination? Electrophysiological recordings and Ca++ imaging studies demonstrate that each immature retina generates correlated propagating waves (Fig. 19.17), which have no preferred direction of propagation and that occur periodically, about once a minute. It is possible that retinal waves contain temporal and spatial cues that guide activity-dependent refinement of retinogeniculate connections. For example, because waves are generated independently in each retina, activities from the two eyes are unlikely to be coincident, while nearby retinal ganglion cells in the same eye would tend to have similar spontaneous activity patterns. Asynchrony between the inputs of the two eyes could account for the segregation of inputs into different eye-specific layers in the thalamus. Since ocular dominance column formation is initiated before birth, the spontaneous patterns of activity from the two eyes could be responsible for the eye-specific pathways throughout the visual system. Moreover, because the waves ensure that nearby retinal ganglion cells are better synchronized than more distant cells, geniculate neurons are able to gauge neighbor relationships in the retina by their sequential activation. This feature could be useful for refinements of the retinotopic map in both thalamus and cortex. Pharmacological blockade of retinal waves during the period of eye-specific segregation prevents the emergence of these layers, suggesting that activity from the retinas is involved (Wong, 1999). However, these activity patterns must be only part of the story: the laminar organization of the lateral geniculate is stereotyped from animal to animal, suggesting that other developmental mechanisms are also at play.
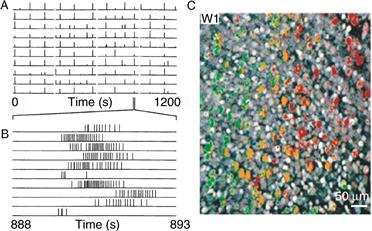
Figure 19.17 Immature retinal ganglion cells show correlated patterns of activity. (A) Using an array of extracellular recordings, rhythmic bursts of action potentials (indicated by vertical lines) are synchronized between neighboring retinal ganglion cells before eye opening in ferret. (B) Action potential bursts expanded in time scale show that each burst corresponds to ten or more action potentials. (C) Using calcium indicators it has been possible to observe waves of neuronal activity in immature retinal ganglion cells (pseudo-colored image). In this example, a wave of neuronal activity propagates through the retina. Pseudo-colored cells indicating the temporal firing pattern of retinal ganglion neurons; cells in green fire before yellow ones, and lastly red cells.
(A, B) from Meister, Wong, Baylor, and Shatz (1991). (C) from Wong (1999).
Cerebellum
A particularly clear example of synapse elimination in the developing CNS is the climbing fiber input onto cerebellar Purkinje cells. Climbing fibers are the terminals of the axons arriving from inferior olive neurons that form strong synaptic connections to Purkinje cells. In adults only one climbing fiber innervates each Purkinje cell. That input might contain 500 synaptic boutons that tightly invest the large ascending proximal dendrite and its major branches. Immature climbing fibers, on the other hand, form fewer synapses, mostly on the Purkinje cell soma, and many climbing fibers project to each Purkinje cell in the first postnatal week (in rodents). The transition from multiple innervation to single innervation of individual Purkinje cells occurs at the same time there is a change in the number of Purkinje cells innervated by each climbing fiber. This “neural unit” shrinkage is seemingly quite analogous to the reduction in the size of motor units in the peripheral nervous system. Thus one olivocerebellar axon may give rise to branches that innervate more than 100 Purkinje cells during the first postnatal week, but over the next several weeks its projection is trimmed to only about 7 Purkinje cells (Fig. 19.18), albeit these connections are much more powerful.
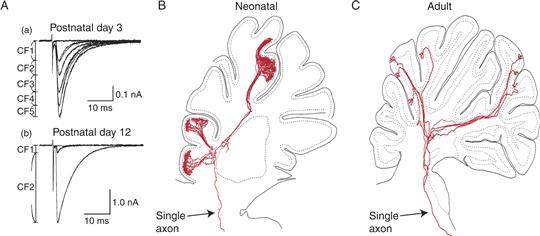
Figure 19.18 Synapse elimination and axonal pruning of climbing fibers in the neonatal period. (A) Recordings from Purkinje cells while olivocerebellar axons are stimulated in the inferior olive show functional evidence of synapse elimination. At birth, each Purkinje cell is innervated by several different climbing fibers, and as the strength of stimulation is increased, additional inputs are recruited (compare with Figs. 19.1C and 19.15B). The average number of climbing fibers innervating each cerebellar Purkinje cell, in the rat, decreases gradually as the animal matures until most are singly innervated. (B, C) Reconstructions of the trajectory of single neonatal (B) and adult (C) olivocerebellar climbing fiber axons. Both neonatal and adult axons terminate in several separate lobules in the hemisphere. However, neonatal olivocerebellar axons have many more branches than those in adult axons and presumably innervate many more Purkinje cells than those in adult animals. After pruning is complete, each axon gives rise to ~7 climbing fibers that each singly innervates a different Purkinje cell.
(A) Adapted from Hashimoto and Kano (2003). (B, C) Modified from Sugihara (2005).
It has long been appreciated that the loss of climbing fiber inputs depends on the presence of parallel fiber innervation from granule cells of the distal part of the Purkinje cell arbor. Elimination of granule cell inputs to Purkinje cells by X irradiation, viral infection, or in mutants such as reeler, weaver, and staggerer results in a higher incidence of Purkinje cells that are multiply innervated by climbing fibers in adulthood. Some studies suggest that the activity of the parallel fiber input is the important parameter. Perturbation of activity along the parallel fiber–Purkinje cell pathway in mGluR1 and GluRδ2 knockout mice or application of NMDA receptor antagonists all inhibit the elimination of climbing fibers (Hashimoto & Kano, 2005). In addition, disruption of one calcium binding kinase (PKC gamma) appears to selectively prevent climbing fiber elimination (Hashimoto & Kano, 2005). Although the mechanism by which this kinase alters synapse elimination is not known, these animals recently have been shown to have a profound deficit in vestibulo-ocular reflex (VOR) motor learning but not other kinds of cerebellar learning (Kimpo & Raymond, 2007). These results imply that synapse elimination may be important in generating the circuitry for some kinds of adult learning. As we will mention later, however, synapse elimination itself may be a form of learning.
Summary
In many parts of the central and peripheral nervous system, the divergence and convergence of synaptic circuits are decreased. A popular hypothesis, albeit still somewhat perplexing, is that neural activity differences between axons underly synapse elimination. This hypothesis has driven researchers to try many kinds of experiments to test electrical activity’s role. At present, the precise way in which electrical activity exerts an influence on the synapse elimination process remains a central and unresolved question.
Is Synapse Elimination Strictly a Developmental Phenomenon?
In this chapter, we presented evidence to demonstrate that synapse elimination is a powerful force that can refine synaptic circuits in young animals, based on interneuronal competition. Is there any reason to think it is more than a strictly developmental phenomenon? The most important form of adult plasticity must certainly be memory. Might synapse elimination have something to do with memory? A number of neurobiologists, including Kandel (1967), Toulouse, Dehaene, and Changeux (1986), and Edeleman (1988), have explicitly made arguments for selection (as opposed to instruction) as potentially playing an important role in learning. The idea is that in the brain synaptic circuitry exists a priori for many things that may ultimately be learned, so that learning might occur by the selection of synaptic pathways that already exist rather than construction of new circuits. Although such selection could occur by increasing the strength of one set of synaptic interconnections or weakening of others, it could also occur by completely eliminating some circuits. It is important to emphasize the distinction between plasticity that alters the strengths of existing connections and the more extreme kind of plasticity, analogous to the developmental synaptic processes described here, that causes permanent eradication of an axon’s input to particular postsynaptic cells.
Because postsynaptic cells appear to be the intermediary in synaptic competition leading to axonal removal (see earlier), once an axon’s synaptic drive to a postsynaptic cell is removed, it can no longer have any influence on the synaptic connections of the other axons that remain connected. Complete loss of influence following synaptic disconnection is thus a plausible explanation for the finite length of critical periods. For example, once all the inputs driven by an eye deprived of vision are eliminated, return of visual experience in that eye can no longer cause a shift in ocular dominance columns if that shift is mediated via activation of postsynaptic cells. The same argument could also be made for memory. Memories have a kind of indelibility that prevents more recent unrelated memories from “overwriting” prior ones. Input elimination is an attractive means of ensuring indelibility because by eliminating competing (i.e., asynchronously firing) inputs, a circuit becomes sheltered from disruption by different activity patterns.
A model of memory based on this kind of synapse elimination, however, would require that axonal inputs continue to be eliminated in the adult brain. That critical periods in the visual system are strictly developmental can be used as an argument against the idea that these kinds of changes may underlie adult memory. On the other hand, critical periods in the visual system tend to be prolonged in proportion to their distance from the retinal input. For example, critical periods for higher visual processing areas occur later in development than in those areas that are more proximal in the visual pathway. The loss of overlapping connections is known to occur prenatally within thalamic circuitry well before segregation in layer IV primary visual cortex, and higher anatomical levels in the visual cortex that receive input from layer IV segregate out later than layer IV. A possible explanation for this sequential crystallization of brain regions may be that synapse elimination can occur only when a cohort of synchronous inputs work together to drive the elimination of competing inputs. Such a collection of synchronously active neurons requires that the presynaptic input to these cells has itself sorted out. It also is the case that the length of the critical periods for vision are vastly longer in humans than other mammals, as is the rest of our neotenic development. For example, whereas our closest animal relatives finish the critical period for monocular deprivation by 7 months of age, in humans, monocular deprivation can affect visual acuity even in children 6 to 7 years old.
Summary
We would not like to give the impression that naturally occurring synapse elimination at developing systems is the equivalent of learning and memory. But, as neurobiologists who have studied this phenomenon and mulled these ideas over for many years, we have come to the conclusion that permanent loss of axonal input is an attractive mechanism for information storage. Whether our bias is reasonable based on the data or rather due to structural elimination of competing hypotheses from our brains, we do not know.
References
1. Antonini A, Fagiolini M, Stryker MP. Anatomical correlates of functional plasticity in mouse visual cortex. Journal of Neuroscience. 1999;19:4388–4406.
2. Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993;260:1819–1821.
3. Balice-Gordon RJ, Chua CK, Nelson CC, Lichtman JW. Gradual loss of synaptic cartels precedes axon withdrawal at developing neuromuscular junctions. Neuron. 1993;11:801–815.
4. Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372:519–524.
5. Bingol B, Wang CF, Arnott D, Cheng D, Peng J, Sheng M. Autophosphorylated CaMKII alpha acts as a scaffold to recruit proteasomes to dendritic spines. Cell. 2010;140:567–578.
6. Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44:651–661.
7. Boeke J. Nerve endings, motor and sensory. Vol. 1 New York: Hafner Press; 1932.
8. Brown MC, Jansen JK, Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. Journal of Physiology. 1976;261:387–422.
9. Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434.
10. Buffelli M, Busetto G, Cangiano L, Cangiano A. Perinatal switch from synchronous to asynchronous activity of motoneurons: link with synapse elimination. Proceedings of National Academy of Sciences of the United States of America. 2002;99:13200–13205.
11. Busetto G, Buffelli M, Tognana E, Bellico F, Cangiano A. Hebbian mechanisms revealed by electrical stimulation at developing rat neuromuscular junctions. Journal of Neuroscience. 2000;20:685–695.
12. Callaway EM, Soha JM, Van Essen DC. Competition favouring inactive over active motor neurons during synapse elimination. Nature. 1987;328:422–426.
13. Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–966.
14. Colman H, Nabekura J, Lichtman JW. Alterations in synaptic strength preceding axon withdrawal. Science. 1997;275:356–361.
15. Constantine-Paton M, Law MI. Eye-specific termination bands in tecta of three-eyed frogs. Science. 1978;202:639–641.
16. Costanzo EM, Barry JA, Ribchester RR. Co-regulation of synaptic efficacy at stable polyneuronally innervated neuromuscular junctions in reinnervated rat muscle. Journal of Physiology. 1999;521(Pt 2):365–374.
17. Costanzo EM, Barry JA, Ribchester RR. Competition at silent synapses in reinnervated skeletal muscle. Nature Neuroscience. 2000;3:694–700.
18. Crowley JC, Katz LC. Early development of ocular dominance columns. Science. 2000;290:1321–1324.
19. Ding M, Chao D, Wang G, Shen K. Spatial regulation of an E3 ubiquitin ligase directs selective synapse elimination. Science. 2007;317:947–951.
20. Eaton BA, Davis GW. Synapse disassembly. Genes & Development. 2003;17:2075–2082.
21. Edeleman G. The theory of neuronal group selection New York: Basic Books; 1988.
22. Erisir A, Dreusicke M. Quantitative morphology and postsynaptic targets of thalamocortical axons in critical period and adult ferret visual cortex. Journal of Comparative Neurology. 2005;485:11–31.
23. Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186.
24. Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51.
25. Gan WB, Lichtman JW. Synaptic segregation at the developing neuromuscular junction. Science. 1998;282:1508–1511.
26. Gorio A, Marini P, Zanoni R. Muscle reinnervation—III Motoneuron sprouting capacity, enhancement by exogenous gangliosides. Neuroscience. 1983;8:417–429.
27. Hashimoto K, Kano M. Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron. 2003;38:785–796.
28. Hashimoto K, Kano M. Postnatal development and synapse elimination of climbing fiber to Purkinje cell projection in the cerebellum. Neuroscience Research. 2005;53:221–228.
29. Hebb DO. The organization of behavior New York: Wiley; 1949.
30. Hensch TK. Critical period regulation. Annual Review of Neuroscience. 2004;27:549–579.
31. Hensch TK. Critical period plasticity in local cortical circuits. Nature Reviews Neuroscience. 2005;6:877–888.
32. Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508.
33. Horton JC, Adams DL. The cortical column: A structure without a function. Philosophical Transactions of Royal Society of London Series B, Biological Sciences. 2005;360:837–862.
34. Horton JC, Hocking DR. An adult-like pattern of ocular dominance columns in striate cortex of newborn monkeys prior to visual experience. Journal of Neuroscience. 1996;16:1791–1807.
35. Horton JC, Hocking DR. Effect of early monocular enucleation upon ocular dominance columns and cytochrome oxidase activity in monkey and human visual cortex. Visual Neuroscience. 1998;15:289–303.
36. Hubel DH, Wiesel TN. Receptive fields of cells in striate cortex of very young, visually inexperienced kittens. Journal of Neurophysiology. 1963;26:994–1002.
37. Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philosophical Transactions of Royal Society of London Series B, Biological Sciences. 1977;278:377–409.
38. Issa NP, Trachtenberg JT, Chapman B, Zahs KR, Stryker MP. The critical period for ocular dominance plasticity in the Ferret’s visual cortex. Journal of Neuroscience. 1999;19:6965–6978.
39. Jackson H, Parks TN. Functional synapse elimination in the developing avian cochlear nucleus with simultaneous reduction in cochlear nerve axon branching. Journal of Neuroscience. 1982;2:1736–1743.
40. Jennings C. Developmental neurobiology Death of a synapse. Nature. 1994;372:498–499.
41. Kalinovsky A, Boukhtouche F, Blazeski R, et al. Development of axon-target specificity of ponto-cerebellar afferents. PLoS Biology. 2011;9:e1001013.
42. Kandel E. Cellular studies of learning New York: Rockefeller University Press; 1967.
43. Kasthuri N, Lichtman JW. The role of neuronal identity in synaptic competition. Nature. 2003;424:426–430.
44. Keech RV, Kutschke PJ. Upper age limit for the development of amblyopia. Journal of Pediatric Ophthalmology & Strabismus. 1995;32:89–93.
45. Keller-Peck CR, Walsh MK, Gan WB, Feng G, Sanes JR, Lichtman JW. Asynchronous synapse elimination in neonatal motor units: studies using GFP transgenic mice. Neuron. 2001;31:381–394.
46. Kimpo RR, Raymond JL. Impaired motor learning in the vestibulo-ocular reflex in mice with multiple climbing fiber input to cerebellar Purkinje cells. Journal of Neuroscience. 2007;27:5672–5682.
47. Laskowski MB, Colman H, Nelson C, Lichtman JW. Synaptic competition during the reformation of a neuromuscular map. Journal of Neuroscience. 1998;18:7328–7335.
48. Li Z, Jo J, Jia JM, Lo SC, Whitcomb DJ, Jiao S. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–871.
49. Lichtman JW. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. Journal of Physiology. 1977;273:155–177.
50. Lichtman JW, Balice-Gordon RJ. Understanding synaptic competition in theory and in practice. Journal of Neurobiology. 1990;21:99–106.
51. Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278.
52. Lichtman JW, Purves D. The elimination of redundant preganglionic innervation to hamster sympathetic ganglion cells in early post-natal life. Journal of Physiology. 1980;301:213–228.
53. Lichtman JW, Wilkinson RS, Rich MM. Multiple innervation of tonic endplates revealed by activity-dependent uptake of fluorescent probes. Nature. 1985;314:357–359.
54. Livingstone MS. Ocular dominance columns in New World monkeys Journal of Neuroscience. 1996;16:2086–2096.
55. Lu T, Trussell LO. Development and elimination of endbulb synapses in the chick cochlear nucleus. Journal of Neuroscience. 2007;27:808–817.
56. Mariani J. Elimination of synapses during the development of the central nervous system. Progress in Brain Research. 1983;58:383–392.
57. Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943.
58. Personius KE, Balice-Gordon RJ. Loss of correlated motor neuron activity during synaptic competition at developing neuromuscular synapses. Neuron. 2001;31:395–408.
59. Personius KE, Chang Q, Mentis GZ, O’Donovan MJ, Balice-Gordon RJ. Reduced gap junctional coupling leads to uncorrelated motor neuron firing and precocious neuromuscular synapse elimination. Proceedings of National Academy of Sciences of the United States of America. 2007;104:11808–11813.
60. Redfern PA. Neuromuscular transmission in new-born rats. Journal of Physiology. 1970;209:701–709.
61. Ribchester RR. Cartels, competition and activity-dependent synapse elimination. Trends in Neurosciences. 1992;15:389 author reply 390–381.
62. Ribchester RR, Barry JA. Spatial versus consumptive competition at polyneuronally innervated neuromuscular junctions. Experimental Physiology. 1994;79:465–494.
63. Ridge RM, Betz WJ. The effect of selective, chronic stimulation on motor unit size in developing rat muscle. Journal of Neuroscience. 1984;4:2614–2620.
64. Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. 1990;5:745–756.
65. Smith SL, Trachtenberg JT. Experience-dependent binocular competition in the visual cortex begins at eye opening. Nature Neuroscience. 2007;10:370–375.
66. Stent GS. A physiological mechanism for Hebb’s postulate of learning. Proceedings of National Academy of Sciences of the United States of America. 1973;70:997–1001.
67. Sugihara I. Microzonal projection and climbing fiber remodeling in single olivocerebellar axons of newborn rats at postnatal days 4–7. Journal of Comparative Neurology. 2005;487:93–106.
68. Tavazoie SF, Reid RC. Diverse receptive fields in the lateral geniculate nucleus during thalamocortical development. Nature Neuroscience. 2000;3:608–616.
69. Thompson WJ. Activity and synapse elimination at the neuromuscular junction. Cell and Molecular Neurobiology. 1985;5:167–182.
70. Toulouse G, Dehaene S, Changeux JP. Spin glass model of learning by selection. Proceedings of National Academy of Sciences of the United States of America. 1986;83:1695–1698.
71. Walsh MK, Lichtman JW. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron. 2003;37:67–73.
72. Wang GY, Liets LC, Chalupa LM. Unique functional properties of on and off pathways in the developing mammalian retina. Journal of Neuroscience. 2001;21:4310–4317.
73. Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591.
74. Wong RO. Retinal waves and visual system development. Annual Review of Neuroscience. 1999;22:29–47.