Chapter 28
The Spinal and Peripheral Motor System
Most coordinated movements involve the use of several muscles, each with a precisely orchestrated timing and pattern of activation and relaxation. Contraction of an individual muscle is controlled by a pool of motor neurons that innervate that muscle. Patterned contraction of several muscles requires coordination between pools of motor neurons. For many movements, interneurons in the spinal cord provide the means for coordinating activity of different motor neuron pools. A key feature of spinal interneurons is their convergent and divergent connections that allow them to integrate signals from different sources. Interneurons that receive inputs from descending motor systems and sensory afferents, for example, can update a voluntary command for limb movement to incorporate feedback from the moving limb. Spinal interneurons also form connections with other spinal interneurons to create networks that generate rhythmic patterns of excitatory and inhibitory activity. These networks play a key role for organizing rhythmic, repetitive movements such as scratching or locomotion. In this chapter, we will use locomotion as an example of a motor behavior to illustrate how the neural elements of the spinal cord and peripheral neuromuscular system operate together to carry out a movement. As details of the motor neurons, spinal interneurons, muscles, and sensory afferents are presented, the primary focus will be on mammals, but variations that occur in other vertebrates and in invertebrates are highlighted in Box 28.1.
Box 28.1 Species Diversity in Neuromuscular Systems
Not all animals control muscle contraction in the same way as mammals. Differences are found in the proteins and structure of the muscle fiber, the number of neuromuscular junctions on each muscle fiber, the neurotransmitters used at the neuromuscular junction, and the number of motor neurons contacting each muscle fiber. Major differences are found in invertebrates, which often have evolved adaptations that suit their movement patterns or constraints imposed by their environments.
Some invertebrates require muscles that generate great forces or provide mechanical stiffness. One solution used by mussels, the nematode C. elegans, and the fruit fly Drosophila is the incorporation of molecules of paramyosin into thick filaments, so that additional cross-bridges can be formed. Mussel retractor muscles also have a specialized catch property, in which cross-bridges are tightly bound with low energy expenditure until the catch state is released by neurotransmitter action that leads to phosphorylation of twitchin, a titin-like molecule. Very slow movements can be achieved by long sarcomeres, since Ca2+ diffusion throughout the sarcomere takes longer. In mammalian muscle fibers, sarcomeres are generally 2–3 microns long, although longer sarcomeres are occasionally found in muscles that stiffen but do not twitch, such as the tensor tympani muscle of the cat eardrum. In contrast, claw muscles of crabs may have sarcomeres from 6 to 18 microns, and one marine worm was found to have sarcomere lengths of 30 microns.
Most mammalian muscles usually contain a mixture of muscle fiber types, but some animals have distinct muscles for fast and slow movements. Trout, for example, use red muscles composed of slow twitch fibers for sustained swimming and white, fast twitch muscles for leaping and rapid bursts of speed.
Mammals and birds use acetylcholine as the transmitter at the neuromuscular junction. So does C. elegans, but many other invertebrates use glutamate, including arthropods such as Drosophila, locust, and crayfish. Co-release of glutamate and peptides occurs at some neuromuscular junctions, such as the peptide FMRF-amide in the sea snail Aplysia. Crustacean muscles have separate excitatory and inhibitory neuromuscular junctions that use glutamate and GABA.
In mammals, most muscle fibers are innervated by a single motor neuron, with a few exceptions, such as laryngeal muscles and spindles. Innervation by more than one motor neuron is seen in some avian muscles, and many invertebrate muscles. The motor neurons may merely have an additive function, as in Drosophila, where recruitment of motor neurons produces graded contractions of the muscle. In other animals, the different motor neurons may play distinct roles. The locust jumping muscle has a dual arrangement, with one motor neuron that produces a rapid and powerful excitation and another that produces graded excitation and contraction.
Lastly, the central control of motor neurons differs. In mammals, a motor neuron action potential leads inevitably to a muscle fiber action potential. In crayfish, presynaptic inhibition of motor terminals allows modulation at a more distant site. Structural specializations, such as electrical synapses onto motor neurons and gap junctions between motor neurons, often occur in invertebrates, but generally are found only during development in mammals. The distributed structure of invertebrate systems and rich palette of ion channels expressed by motor neurons is favorable to the formation of motor pattern generating circuits.
Mary Kay Floeter
Locomotion is A Cycle
Locomotion is the act of moving from place to place. Walking, swimming, and flying are modes of locomotion. All modes of locomotion consist of a cycle of patterned and rhythmic activity of different muscle groups. Locomotion can be described by the phase relationships between limbs and between muscle groups during the cycle. Nonlimbed vertebrates such as lampreys swim by alternately contracting and relaxing trunk muscles on the two sides of the body; the sides are in opposite phases of the cycle. Along the length of the lamprey’s body, there is a slight phase lag between segments, creating the propulsive wave that moves it forward. In contrast, birds activate their wings synchronously in flight; the limbs are in phase, with no lag.
Stepping, the major form of mammalian locomotion, is a cycle with two phases: stance (the extension phase) and swing (the flexion phase). In people, the stance phase begins when the heel strikes the ground and the leg begins to bear weight. As stance progresses, the weight is gradually shifted forward, rolling from the heel to the ball of the foot. The swing phase begins when the toe leaves the ground and the leg moves forward. To accomplish this motor sequence within one leg, muscles acting across the hip, knee, or ankle are activated in a characteristic pattern (Fig. 28.1). In general, muscles that flex the joint are more active during the swing phase and extensor muscles during the stance phase. However, within each phase, the individual muscles have a more elaborate and finely graded timing and pattern of activation. Stepping can also be performed with different gaits. Gaits can be characterized by the cycle rate, the relative proportion of the cycle spent in swing and stance phases, and the phase relationships between limbs. At slow or moderate walking speeds (i.e., slow cycle rates), the extensor phase is longer than flexion and the extension phases of opposite legs overlap. In a walking gait both feet are in simultaneous contact with the ground for some period during the cycle. As the speed of stepping increases and the cycle is shorter, the duration of the extension phase shortens, with briefer periods of double foot contact. At high speeds where there is no overlap of extension phases between the legs, the gait switches recognizably from walking to running.
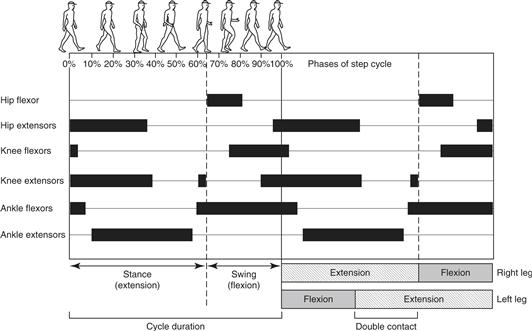
Figure 28.1 Schematic diagram of two step cycles in human locomotion. Drawings at the top illustrate the position of the limbs during one cycle. Dark horizontal bars below the drawings show the timing of activity in flexor and extensor muscles of the right leg. The division of each step cycle into stance (extension) and swing (flexion) phases is indicated at the bottom of the figure. During walking, the stance phases of the two legs overlap, with both feet in contact with the ground.
Central Pattern Generating Circuits
Although we are able to exert voluntary control over locomotion, the basic patterns of movement for locomotion can be generated by the spinal cord itself, without descending (from the brain) or sensory (from the periphery) inputs. The first demonstration came from Graham Brown (1911), who observed that cats in which the spinal cord and dorsal roots were cut were still able to walk on a treadmill as long as their weight was supported. Because there was no sensory feedback or descending input, he suggested that the rhythmic activity in the hindlimb muscles must be generated by a motor program within the spinal cord. The motor program, also known as a central pattern generator (CPG), needed to explain three important features of locomotion: first, its rhythmic and cyclical occurrence; second, coordinated alternation between flexor and extensor activity within the same limb; and third, alternation between opposite limbs. Brown proposed that the CPG consisted of two mutually inhibitory “half-centers,” both under tonic excitation: one half-center drove muscles that flexed the legs and the other drove muscles that extended the legs. The mutual inhibition between the two half-centers would cause activity to oscillate between them, producing alternating limb flexion and extension.
CPGs are now thought to consist of networks of neurons that are synaptically or electrically connected, with cellular properties that generate rhythmic firing. CPGs are found in many areas of the central nervous system and are responsible for different types of patterned rhythmic activities such as breathing, chewing, swallowing and walking. The identification and characterization of the neurons that comprise the locomotor CPG in the spinal cord is an active area of research that will be discussed later in this chapter.
Sensory Contributions to Movement
During a movement, the sensory afferents from muscles, joints, and skin will each be activated in a distinct way, reflecting how that movement displaces joints, produces pressure on regions of skin, and stretches individual muscles. For rhythmic movements such as walking, these recurring “multisensory” feedback signals often reinforce activity of CPG circuits. However, when a perturbation occurs during the movement, the resulting sensory signal may evoke a reflex motor response that alters the movement. Many of these reflex responses adapt the movement in an appropriate way to proceed under the changed environmental conditions. Most sensory afferents produce these reflex effects through interneuronal circuits. (The stretch reflex is an exception.) The excitability of many of these interneuronal circuits is usually modulated during movement, allowing reflexes to be suppressed or enhanced at different phases. CPGs are one source of this phase modulation. Many reflexes can also be elicited when the organism is not moving, and in these situations the reflex tends to exhibit a more stereotyped motor pattern.
Connecting the Spinal Cord to the Periphery
Ultimately, movement is carried out by muscles, and the motor commands from the central nervous system must be transmitted to motor neurons that innervate the muscles. The organization of motor neurons into pools, and the manner in which motor neurons’ properties are matched to contractile and metabolic properties of the muscle fibers simplify many of the variables needed to be controlled to produce smooth movements with precise amounts of force. Feedback from sensory receptors of the muscle play a key role in monitoring the force and stretch of the muscle. This section will cover some of the basic properties of motor neurons, muscle, and muscle receptors.
Motor Neurons
Motor neurons are located in the ventral horn of the spinal cord. The pool of motor neurons that innervates an individual muscle forms an elongated column that typically extends over two or three spinal cord segments. Intermingled among the large α-motor neurons that innervate skeletal muscle fibers are smaller γ-motor neurons, which innervate muscle spindles, as well as β-motor neurons, which dually innervate skeletal muscle and spindles. The α-motor neurons are the largest neurons in the spinal cord, with myelinated axons that exit the spinal cord through the ventral roots and travel in peripheral nerves to innervate muscles. Their axons also give off small branches within the spinal cord, called recurrent collaterals, that synapse on inhibitory interneurons, called Renshaw cells (Alvarez & Fyffe, 2007), and other motor neurons. The extensive dendritic tree of motor neurons extends into the dorsal and ventral horns, receiving thousands of synaptic inputs from excitatory and inhibitory neurons (Fig. 28.2). At the neuromuscular junction, the synapse between the motor axon and the muscle fiber, acetylcholine is the transmitter used by mammals, but other transmitters, such as the peptide CGRP or glutamate/aspartate (Mentis et al., 2005; Nishimaru, Restrepo, Ryge, Yanagawa, & Kiehn, 2005), coexist in many motor neurons and may play a particular role during development and at the central synapses formed by recurrent collaterals.
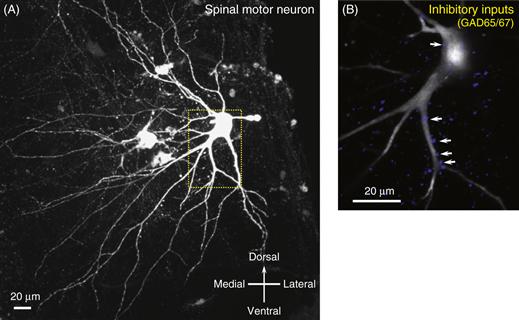
Figure 28.2 Motor neuron from a neonatal mouse spinal cord that has been filled with biocytin through an intracellular electrode and visualized by immunocytochemistry. (A) The extensive dendritic arbor is directed into the dorsal, medial, and ventral gray matter of the spinal cord, where dendrites receive thousands of synapses from interneurons, sensory afferents, and descending pathways. (B) Double staining for a marker of GABA ergic inhibitory terminals (GAD65/67) shows some of the inhibitory synapses along the proximal dendrites and cell body.
Muscle Contraction
Movement is achieved by the contraction of muscles that are attached to the skeleton or soft tissues, such as muscles producing facial expressions. A skeletal muscle is made up of thousands of individual muscle fibers. Each muscle fiber is a multinucleated cell, a few centimeters long, that contains the contractile proteins, actin and myosin, arranged into thick and thin filaments in repeating units called sarcomeres (Fig. 28.3). The myosin molecule head region contains an ATP ase enzymatic activity that is sensitive to levels of intracellular calcium. Myosin heads are loosely bound to actin at rest, but when intracellular calcium rises, the cross-bridge strengthens, causing myosin to break down ATP. When this occurs, the cross-bridge loosens, and the myosin head swivels along the actin filament to another binding site. The repetition of this swiveling movement in the presence of elevated intracellular calcium and ATP causes thick and thin filaments to slide along each other and shorten the sarcomere length. Shortening of sarcomeres shortens the muscle, or if the muscle is carrying a heavy load, the muscle may only stiffen in an isometric contraction.
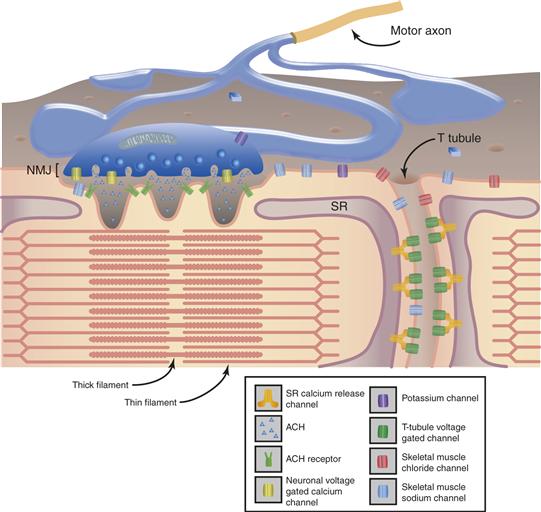
Figure 28.3 Diagram of the structure of the muscle showing the relationship between the thick and thin filaments that form the sarcomere, the sarcoplasmic reticulum (SR), and the location of the ion channels of the motor axon, neuromuscular junction, and muscle membrane.
With permission, modified from Cooper and Jan (1999).
The process of muscle contraction begins with an action potential in the motor axon that causes release of acetylcholine at the neuromuscular junction. In mammals, normally every spike in the motor axon produces an action potential in the muscle fiber because an excess amount of acetylcholine is released (Box 28.2). In adult mammals, each skeletal muscle fiber has one neuromuscular junction innervated by one motor neuron. Muscle fiber action potentials lead to the activation of voltage-gated calcium channels in the membrane that interact with calcium release proteins in the sarcoplasmic reticulum, an organelle in the muscle fiber that stores and releases calcium near the sarcomeres. This interaction couples excitation to muscle contraction. Other proteins in the sarcoplasmic reticulum reuptake calcium, leading to relaxation.
Box 28.2 Myasthenia and Myasthenic Disorders
Normally every action potential of the motor axon releases more than enough transmitter needed to produce a muscle fiber action potential. This occurs because both the amount of transmitter released from the presynaptic motor axon terminal and the density of AChRs on the postsynaptic muscle fiber exceed that needed to produce an end plate potential (EPP) large enough to initiate a muscle fiber action potential. Genetic or acquired diseases that reduce the safety factor of neuromuscular transmission are called myasthenic disorders (Fig. 28.B1). Myasthenic disorders cause weakness that fluctuates with use of the muscle. The most common is the acquired autoimmune disorder myasthenia gravis, caused by antibodies that bind to AChRs at the neuromuscular junction. The antibodies interfere with ACh binding and lead to internalization of AChRs and, ultimately, the loss of junctional folds. The reduced numbers of functioning AChRs produce a smaller EPP that fails intermittently to trigger a muscle fiber action potential. However, in myasthenia gravis the presynaptic terminal remains normal, such that a short burst of stimulation will produce facilitation of transmitter release, increasing the concentration of ACh in the cleft and transiently improving neurotransmission. Acetylcholinesterase inhibitors provide symptomatic improvement by blocking the breakdown of ACh, allowing a longer effect on residual receptors. Another autoimmune myasthenic disorder, Lambert–Eaton myasthenic syndrome, is caused by antibodies that bind to presynaptic voltage-gated calcium channels. Less ACh is released at the neuromuscular junction, producing small EPPs that fail to trigger action potentials
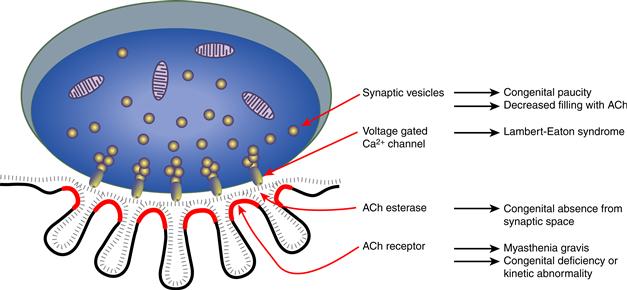
Figure 28.B1 Schematic diagram of a neuromuscular junction showing key components subserving neuromuscular transmission and the myasthenic disorders associated with these components.
Congenital myasthenic syndromes (CMS) are extremely rare, but provide important insights into the function of components of the neuromuscular junction. Postsynaptic CMS stem from a deficiency and/or altered kinetic properties of the AChR. More than 40 different mutations in subunits of the AChR have been described. Most mutations decrease subunit expression or prevent subunit assembly or glycosylation, leading to reduced numbers of functioning AChRs and thereby smaller EPPs. In fast channel CMS, mutations produce decreased binding affinity for ACh, leading to a smaller quantal response and miniature EPP amplitude, without reducing the numbers of AChRs. In contrast, the slow channel CMS stem from mutations that prolong the channel opening episodes. Slow channel mutations cause depolarization block due to temporal summation of the prolonged EPPs. In the long term, cationic overloading of the postsynaptic region leads to destruction of the junctional folds and loss of AChRs. Presynaptic CMS are less common. They are caused by mutations that lead to a paucity of synaptic vesicles or defects in ACh resynthesis or vesicular packaging. As a result, fewer vesicles are released or fewer ACh molecules are contained in each vesicle, and EPPs are smaller. Identifying CMS as involving pre- or postsynaptic components and understanding how these alterations affect neuromuscular transmission are necessary for developing rational therapies for these disorders.
Mary Kay Floeter
Three Basic Muscle Fiber Types
Muscle fibers are classified into types according to their contractile and metabolic properties. Contractile properties refer to the muscle fiber’s speed of contraction and relaxation, and the specific force it can produce. Contractile properties are mainly determined by the isoform of myosin that is expressed by the muscle fiber, particularly the myosin heavy chain gene, although the genes for other proteins associated with the sarcomere and the sarcoplasmic reticulum are expressed in fiber-type specific patterns that match the properties of the myosin isoform. The contractile properties of a muscle fiber are classified as slow twitch or fast twitch. Metabolic properties refer to whether aerobic or anaerobic pathways are used to produce energy (ATP) from fuels. Muscle fibers with abundant mitochondria are able to use oxidative (aerobic) metabolism, which provides relative resistance to fatigue as long as there is a good blood supply. Muscle fibers with few mitochondria rely on stored fuel, such as glycogen, which can more easily be depleted and also produces lactic acid as a byproduct of glycolysis. There are three basic muscle fibers types in adult skeletal muscle. Type I fibers have a slow twitch but have a good capacity for oxidative metabolism. Type IIA fibers have a fast twitch and can use both aerobic and anaerobic metabolism, giving them an intermediate resistance to fatigue. Type IIB fibers produce a fast twitch, but rely mainly on anaerobic glycolysis for their energy. Most muscles contain a mixture of all three fiber types, in proportions that reflect the typical usage of the muscle.
Motor Unit Types
A “motor unit” is defined as one motor neuron and all of the muscle fibers it innervates. In mammals, each muscle fiber is innervated by only one motor neuron, but one motor neuron innervates many muscle fibers as its axon branches in the muscle. However, all muscle fibers of one motor unit are of the same fiber type. Motor units cluster into three basic types based on the three physiological properties of twitch speed, the amount of force produced, and fatigability (Fig. 28.4). Type S motor units are defined by the properties of slow twitch, small amounts of force, and high resistance to fatigue. Type FR motor units are defined by fast twitch, moderately large force, and relative resistance to fatigue. Type FF motor units have fast twitch, produce the highest forces, and fatigue quickly.
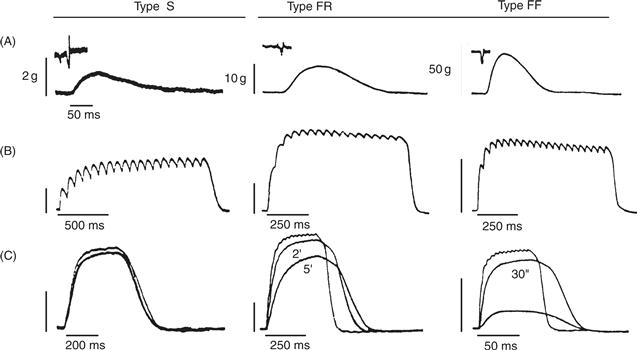
Figure 28.4 The three motor unit types (S, FR, and FF) can be defined experimentally by measuring their contractile properties and fatigability. Panels A–C show recordings of muscle force, with insets in A showing recordings of motor neuron action potentials. Note different time and amplitude calibration scales for each of the motor units. (A) Single twitches produced by one action potential of the motor neuron. (B) Maximal force produced by repetitive stimulation of the motor neuron to produce an unfused tetanus. In addition to differences in maximal force, the “sag” property, a dropping off of tension during maintained stimulation, is seen in FR and FF units. (C) Fatigability is demonstrated by a drop in the tension produced by a single twitch after short periods of activation, as noted. Note that S units show little fatigue, whereas FF units fatigue within 30 s.
Reproduced with permission from Burke, Levine, Tsairis, and Zajac (1973).
The physiological properties of the motor neurons and the muscle fibers they innervate are closely matched. Type S motor neurons innervate Type I fibers. Type S motor neurons have small cell bodies and dendritic arbors, allowing them to be depolarized easily. Afterhyperpolarization following each motor neuron spike promotes a steady rate of firing that allows forces produced by successive twitches of the muscle fibers to summate (Fig. 28.4B). With their steady firing rates and resistance to fatigue, Type S motor units are optimal for the sustained firing needed for postural and tonic movements. Each Type S unit individually contributes only a small amount of force to a muscle’s contraction, but typically Type S units make up a large proportion of the motor units innervating a muscle. In contrast, Type F motor neurons have large cell bodies and dendritic arbors, shorter afterhyperpolarizations and somewhat faster firing rates. Type FR motor neurons innervate Type IIA muscle fibers, and Type FF motor neurons innervate Type IIB muscle fibers. Type FR motor units are optimal for fast, powerful movements. Type FF units are mainly used in movements that require brief bursts of muscle strength. Most muscles contain a mixture of motor unit types, intermingled in a mosaic pattern.
The Size Principle and Orderly Recruitment
Most movements don’t require a muscle to contract at its maximal strength, and only a fraction of the hundred or more motor neurons in the pool that innervates that muscle will need to fire. For most movements, motor units are recruited in an orderly sequence, first described by Henneman (1957), as based on the “size” of the motor neuron. According to the size principle, motor units that produce the smallest amounts of force are the first to begin firing, and motor units that produce larger forces will be progressively recruited as the muscle makes progressively stronger contractions. Many of the anatomical and physiological properties of the motor unit types correlate with measures of size (Table 28.1). These size related properties favor activation of motor unit types in the following order: Type S → Type FR → Type FF. The pool of motor neuron innervating each muscle contains varying proportions of Type S and Type F motor neurons, although generally Type S motor neurons are more abundant.
Table 28.1 Size-Related Properties of Motor Neurons
| Properties that Increase with Size | Properties that Decrease with Size |
| Diameter of soma and axon | Resistance to fatigue |
| Conduction velocity | Ia EPSP amplitude |
| Complexity of axonal collaterals | Input resistance |
| Membrane area, dendritic extent | Membrane resistance |
| Rheobase | Time constant |
| Muscle fiber diameter | Duration of after-hyperpolarization |
| Maximum force output | Twitch contraction time |
| Twitch relaxation time |
Many of the properties that produce orderly activation by size are stable and intrinsic to the motor neuron–cell body diameter, for example. Most extrinsic synaptic inputs also reinforce recruitment according to the size principle. This occurs because a synaptic current will produce a larger postsynaptic potential in a smaller, compact neuron with a higher input resistance. Because most sources of synaptic input are distributed uniformly to a motor pool, EPSP or IPSP amplitudes correlate with the motor neuron size—larger in Type S motor neurons than Type F motor neurons. This means, for example, that stretch of a muscle that activates the primary muscle spindle (Ia) afferent will produce the largest Ia EPSPs in Type S motor neurons, and these will be the first units recruited to fire in the stretch reflex. In the less common situation in which synaptic inputs are not uniformly distributed to every motor neuron in the pool, alternative recruitment sequences are possible.
Stronger muscle contractions are produced not only by recruitment of additional motor units, but also by increasing the firing rates of motor units that are already actively firing. The relative use of recruitment and rate modulation to maintain a desired profile of force varies according to the muscle and the level of force. In some muscles, such as the small hand muscles, several motor units begin firing at low levels of force, and fine gradations of force are controlled by modulating their firing rates. In other muscles, recruitment and rate coding occur together in a more balanced fashion throughout the working force range of the muscle.
Muscle Afferents
Muscles contain two kinds of specialized sensory receptors that provide feedback to the spinal cord and brain. Muscle spindles sense muscle stretch or length, and Golgi tendon organs (GTOs) sense the load upon the muscle (Fig. 28.5). The spindle consists of an encapsulated bundle of modified muscle fibers, called intrafusal fibers, that lie within the muscle in parallel to the skeletal muscle fibers. The spindle receives both sensory and motor innervation. Sensory innervation is supplied by one primary afferent, the Ia fiber, and several secondary, group II afferents. Ia afferents are more sensitive to quick stretches, such as a tendon tap or vibration, and group II afferents maintain a firing rate proportional to the level of sustained stretch. Intrafusal muscle fibers are innervated by γ-motor neurons and β-motor neurons. The motor innervation of intrafusal fibers adjusts the tension of the spindle to compensate for changes in muscle length when a muscle contracts and shortens. When the muscle shortens the spindle can become momentarily slack, causing a pause in spindle afferent firing. However, during most voluntary movements, α and γ-motor neurons are co-activated such that the sensitivity of the spindle is maintained during the muscle shortening. Nevertheless, because γ-motor neurons have a separate innervation of intrafusal muscle fibers, the nervous system can separately adjust the sensitivity of the spindle for different types of movement.
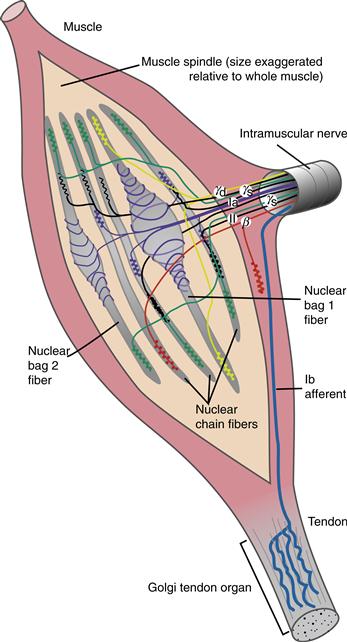
Figure 28.5 Structure and innervation of muscle spindles and Golgi tendon organs. The sensory innervation of the spindle is through primary (Ia) spindle afferents that innervate both nuclear bag and nuclear chain intrafusal muscle fibers and secondary (II) afferents that innervate nuclear chain fibers. Motor innervation of the spindle is supplied by static and dynamic γ-motor neurons and by β-motor neurons.
Golgi tendon organs lie at the junction of the muscle and tendon, in series with the muscle. GTOs are sensitive to the forces generated by muscle contraction against a load. Each GTO senses the force generated by a small number of motor units that insert onto a local region of the tendon. The axons of the GTOs are called Ib afferents. Like Ia afferents, they are large myelinated axons with fast conduction velocities. Unlike spindles, GTOs do not have motor innervation.
Muscles are innervated by smaller diameter and unmyelinated afferents, called group III and IV afferents, that respond to extracellular substances, such as the acidic substances released during fatiguing exercise. These slowly conducting afferents relay the sense of muscle pain and soreness and produce reflex actions with a relatively slow time course.
Stretch Reflexes
The muscle stretch reflex plays an important role in providing feedback during movements. The stretch reflex consists of a monosynaptic response from the direct connection between Ia afferents and motor neurons, that may be followed by polysynaptic reflex activity. At rest, the stretch reflex can be obtained by a quick stretch, such as by tapping on a muscle tendon, which produces a burst of action potentials in the Ia spindle afferents (Fig. 28.6). In the spinal cord, the Ia afferents synapse on the motor neurons of the muscle containing the spindle (the homonymous motor neurons) and, to a lesser extent, on motor neurons that innervate other muscles (heteronymous motor neurons). The rapid volley of glutamatergic Ia EPSPs caused by a quick stretch can summate in the motor neuron, and if sufficient depolarization occurs, an action potential occurs that produces contraction of the muscle, with a jerk of the limb.
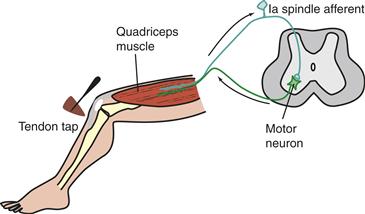
Figure 28.6 Basic circuitry underlying the knee-jerk stretch reflex. Ia spindle afferents from the quadriceps muscle make monosynaptic, excitatory connections on α-motor neurons that innervate the quadriceps.
If the stretch reflex is elicited during movement, the Ia EPSPs will summate with inputs from other sources such as CPGs and descending inputs. Stretch during the phase of movement when the muscle is active will add to other sources of motor neuron depolarization, and the reflex motor response will enhance the ongoing contraction. Stretch of the muscle when it is out of its active phase will produce a smaller motor response, if any. In this way, the spatial summation of postsynaptic potentials contributes to phase modulation of the stretch reflex. Additionally, there is modulation of the stretch reflex through presynaptic inhibition of the Ia afferents during movement (Rudomin, 2009). This presynaptic inhibition is produced by GABA-ergic spinal interneurons that synapse on the Ia afferent terminals, forming axo-axonic synapses (Betley et al., 2009). Presynaptic inhibition reduces the release of glutamate from Ia afferents, leading to smaller Ia EPSPs in the motor neuron, but it does not affect the size of other inputs to the motor neuron. The GABAergic interneurons mediating presynaptic inhibition are strongly activated by antagonist Ia afferents, that is, from spindles of muscles that produce the opposite movement, as well as by CPGs and descending inputs. Thus, presynaptic inhibition of a muscle’s stretch reflex is strongest when the muscle contraction is out of phase with the movement cycle, when its antagonist muscle is contracting.
Summary
Motor neurons are highly diverse in terms of their morphology, connectivity, and functional properties and differ significantly in their response to disease. There are two main types of motor neuron disease, amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (Box 28.3). Motor neurons innervating different muscles have recently been shown to degenerate in a preferential manner.
Muscles are innervated by motor neurons in the ventral horn of the spinal cord. The motor unit consists of one motor neuron and all the muscle fibers it innervates. Physiological properties of each motor neuron match the contractile and metabolic properties of its muscle fibers. Type S motor units have a slow twitch, high aerobic capabilities, and fatigue resistance. These smaller motor units are the first recruited for contraction and are heavily used for slow, sustained postural movements. Type F motor units innervate muscle fibers with a fast twitch and are recruited for more forceful or ballistic movements. Type FR motor units use aerobic and anaerobic metabolism and are relatively resistant to fatigue. Type FF motor units use anaerobic metabolism and fatigue quickly. In the pool of motor neurons innervating a muscle, motor neurons are recruited in an orderly fashion, beginning with Type S units that generate small amounts of force, followed by units that generate greater forces. As the muscle contracts, changes in its length are detected by spindles, specialized receptors that lie within the muscle. The load on the muscle is detected by a second specialized receptor, the Golgi tendon organ. These sensory receptors of the muscle provide feedback to the spinal cord allowing reflex changes in drive to the muscles.
Spinal Interneuron Networks
So far, this chapter has discussed how the output of the spinal cord produces muscle actions through the motor neurons. This section will focus on spinal interneurons—neurons with a cell body in the spinal cord and an axon that synapses on another spinal neuron. Spinal interneurons have three important functions for motor control: they relay sensory inputs from the periphery that may modulate the motor output, they relay as well as modulate signals from the brain via descending pathways, and they form networks that produce patterned rhythmic activity. It is important to note that some interneurons may participate in all three functions, switching from one role to another during specific movements or carrying on two roles simultaneously.
Historically, interneurons were identified and named by their axonal projections, the location of their soma, their neurotransmitter, or by a physiological effect or reflex that they mediated. Later, populations of neurons were identified by their pattern of activity during movements, visualized using activity-dependent dyes. More recently, molecular biology tools have enabled scientists to identify interneurons based on their gene expression pattern and cell lineage. Molecular markers have been used to identify several classes of interneurons and motor neurons. Four distinct populations of interneurons, designated as V0, V1, V2, and V3, have been identified in the ventral spinal cord by their developmental pattern of expression of transcription factors, proteins that regulate gene expression (Jessell, 2000; Goulding & Pfaff, 2005; Grillner & Jessell, 2009). The “V” denotes the ventral location, and each subclass appears at a distinct position along the dorso-ventral axis (0 most dorsal, 3 most ventral) of the ventral spinal cord (Fig. 28.7). The cellular identities of spinal interneurons are further refined by the emergence of combinations of transcription factors that are expressed in these neurons (Goulding 2009; Alaynick, Jessell, & Pfaff, 2011).
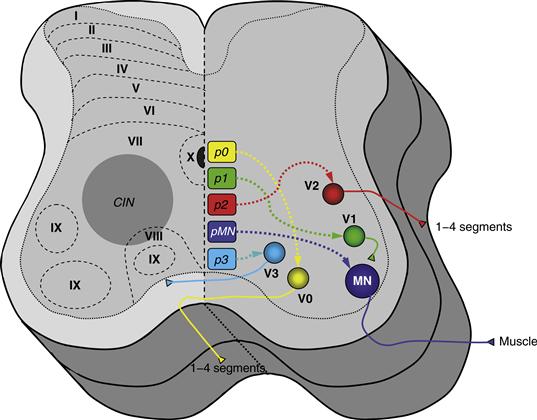
Figure 28.7 Cross-sectional schematic of the spinal cord depicting on the right side the origin of interneuron and motor neuron populations defined in developmental studies using molecular markers for cell identification. The ventral interneurons (V), derived from progenitors (p), are marked as V0–V3 based on the distinct position along the dorso-ventral axis (0 most dorsal, 3 most ventral) of the ventral horn. The left side of the spinal cord depicts the general location of commissural interneurons (CINs) and the Rexed’s laminae. CINs are characterized based on the projection of their axons, as ascending, descending, or both.
Models for Studying the Locomotor CPG Network
At the beginning of the chapter, we noted that the CPG network needed to produce three fundamental features of locomotion: generation of a cyclical rhythm, coordinated alternation between antagonist flexor and extensor muscles, and coordination between limbs. Figuring out what makes up a CPG—the neurons involved, the connections between these neurons, the synaptic interactions, and the contributions from cellular ion channels—is challenging. To tackle this problem, researchers construct models that can be tested and refined by experimental observations. As noted earlier, Brown (1911) first proposed a model with mutually inhibitory half-centers driving flexors and extensors. Grillner (1985) proposed three important modifications: (1) that at each joint, movement in one direction was produced by a “burst generator” unit, a half-center capable of independent rhythmic activity; (2) as in Brown’s model, that flexion and extension at each joint were controlled by reciprocally connected burst generators to form a unit CPG; and (3) that multijoint coordination was achieved by loose or adjustable coupling between the mosaic of unit CPGs controlling each of the joints (Fig. 28.8).
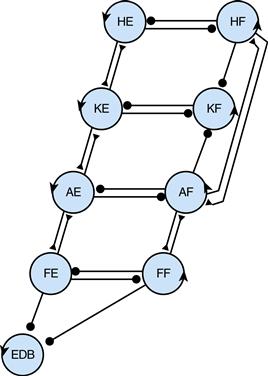
Figure 28.8 Scheme for multijoint coordination within the leg using a mosaic of “unit CPGs.” Burst generators produce movement in one direction, extension or flexion, at the hip (HE, HF), knee (KE, KF), ankle (AE, AF), or foot (FE, FF), with a separate generator for the muscle that lifts the toes (EDB). Burst generators for flexion and extension at each joint are mutually inhibitory, forming a unit CPG that produces alternation. The strength of excitatory (triangles) and inhibitory (circles) connections between unit CPGs can vary to create a variety of different gaits.
Reproduced with permission from Grillner (1985).
Experimental findings have led to further refinements of the proposed organization of the CPG network. For example, the rhythm and timing of the network may be controlled separately from the patterning of the network output. In studies of locomotor rhythms in the neonatal rodent spinal cord, the CPG appears to be distributed among several spinal segments. Studies in the isolated neonatal rodent spinal cord preparation maintained in vitro (Fig. 28.9) show a difference in the rhythmogenic capacity between the rostral (upper) and the caudal (lower) segments of the lumbar spinal cord. The upper lumbar (L1–L3 in rodents) segments seem to be most important for rhythm generation compared to caudal lumbar segments (Cowley & Schmidt, 1997; Tresch & Kiehn, 1999). In experiments using calcium dyes to visualize motor neuron activity, rhythmic signals from different parts of the lumbar (L1, L2) and sacral (S1–S3) segments rose, peaked, and decayed in a rostrocaudal sequence, giving rise to a rostrocaudal “wave” of motor neuron activation during each locomotor cycle (Bonnot, Whelan, Mentis, & O’donovan, 2002). The importance of the upper and mid-lumbar cord for rhythm generation has also been observed in the spinalized cat and is consistent with case reports in humans noting that irritative lesions of the upper lumbar cord produce stepping movements.
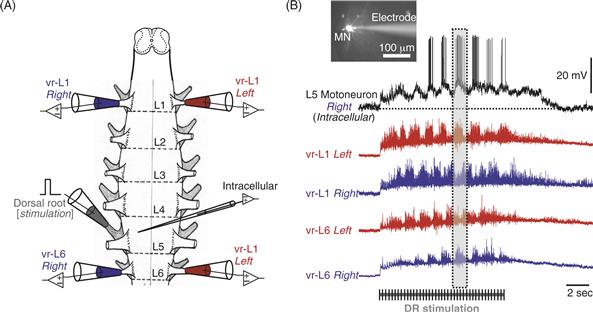
Figure 28.9 (A) Drawing of an isolated neonatal mouse spinal cord used in in vitro experiments. Suction electrodes are placed in the ventral lumbar (L) roots to record motor neuron activity (blue and red) or in the dorsal root (gray) to stimulate sensory afferents. Glass electrodes are used to record intracellularly from individual neurons in the spinal cord. (B) The image shows a motor neuron (MN) filled with a fluorescent dye and visualized with a microscope during intracellular recording. Sustained electrical stimulation of sensory afferents can evoke rhythmic activity in motor neurons (intracellular record) characterized by rhythmic membrane depolarization which reaches the threshold for action potentials. This stimulation also results in alternating rhythmic activity between the left and right side of the spinal cord [compare the activity in L1-left (red) and L1-right (blue)]. It also produces alternating activity between the upper (L1) and lower (L6) segments of the spinal cord [compare the 2 red traces or the 2 blue traces], as highlighted in the dotted box. The duration of afferent stimulation is indicated by the graded bar.
Excitatory Interneurons Responsible for the Rhythm
Maintaining rhythmic bursting in an interneuronal network requires a source of excitatory drive. Although this drive could be provided by tonic excitation, such as from descending pathways, a more robust rhythm is possible if there are excitatory cells within the network that have intrinsic properties that allow rhythmic and repetitive firing (Box 28.4). A few classes of rhythmically active excitatory interneurons have to date been identified in the mouse spinal cord and implicated in central pattern generation. The first type of interneurons is a population of V3-derived interneurons expressing the transcription factor Sim1 (Zhang et al., 2008). These interneurons release glutamate and target motor neurons, as well as V1 and V2 interneurons. The second type of interneurons is a subpopulation of V2 interneurons, termed V2a, which express the transcription factor Chx10 (Crone et al., 2008; Zhong et al., 2010). The Chx10+ interneurons release glutamate and also contact motor neurons directly. Another candidate population of excitatory interneurons was first identified in mice with deletions in the genes for two axon guidance molecules, EphA4 and ephrinB3 (Kullander et al., 2003). These mice had an abnormal hopping pattern of gait. Subsequent study of interneurons expressing EphA4 receptors showed that they were glutamatergic and rhythmically active during locomotor behavior (Butt, Lundfald, & Kiehn, 2005). Most of the interneurons were “last order” interneurons—that is, interneurons that synapse directly on motor neurons. Finally, another interneuron that is rhythmically active during fictive locomotion using isolated spinal cords of mice is the interneuron that expresses the transcription factor Hb9, which is also expressed in the motor neuron lineage during development (Hinckley, Hartley, Wu, Todd, & Ziskind-Conhaim, 2005; Wilson et al., 2005).
Box 28.3 Motor Neuron Diseases
There are several neurological disorders that selectively affect motor neurons. Spinal muscular atrophy (SMA) is a hereditary disorder in which motor neurons develop normally but begin to degenerate not long after birth. Autosomal recessive SMA was described by neurologists long before its cause was known, and several different clinical phenotypes were described. SMA causing weakness in infancy was called Werdnig-Hoffman disease; SMA causing weakness later in childhood was called Kugelberg-Welander disease. Both forms of SMA are caused by a mutation in a gene called “survival of motor neuron” or SMN gene. The SMN protein is widely expressed in neurons and has a role in processing mRNAs. It is not known why motor neurons are more vulnerable to mutations of SMN than other cells. In an X-linked SMA, called Kennedy’s disease or spinal and bulbar muscular atrophy (SBMA), weakness begins in adult life in affected males. Motor neurons innervating facial and jaw muscles are often affected early on. The mutation in SBMA is a triplet repeat expansion in the androgen receptor gene that leads to expression of an abnormal androgen receptor. The androgen receptor is expressed in motor neurons, and over time accumulation of the abnormal receptor leads to degeneration of the motor neuron.
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, is a disease with degeneration not only of the motor neurons, but also of corticospinal neurons of the motor cortex. In most patients with ALS, the disease is not hereditary and its cause is unknown. There are a few hereditary forms of ALS that make up a small percentage of all patients with ALS. Nevertheless, the identification of familial ALS (FALS) genes has provided ideas about pathways that may be responsible for causing sporadic ALS. The first gene mutation identified in FALS encoded the enzyme superoxide dismutase, SOD1. Studies using transgenic mice expressing the mutant SOD1 gene showed that FALS was not caused by loss of enzyme activity, but by abnormal properties of the mutant SOD1 protein. Studies expressing mutant SOD1 in different cell types are revealing that glial and inflammatory cells may also contribute to motor neuron degeneration in FALS (Boillee, Vande Velde, & Cleveland, 2006). The ability to manipulate the expression of mutant genes in animal models promises to provide an invaluable contribution to finding causes and treatment of ALS.
Mary Kay Floeter
Box 28.4 Nonlinear Properties of Motor Neurons and CPG Interneurons
Experimental evidence from animal work indicates that motor neurons can participate actively in generating the rhythmic pattern observed during locomotion. Some motor neuron properties can be turned ON during locomotion due to synaptic inputs which in turn, produce nonlinear input–output relationships.
For example, there is a locomotor-dependent reduction in the threshold for spike generation, resulting in higher motor neuronal excitability. Two other motor neuronal properties susceptible to interact significantly with afferent inputs are the membrane potential oscillations known as locomotor-drive-potentials (LDP) and plateau potentials. LDPs are membrane potentials in motor neurons, in which a wave of depolarization during the active phase alternates with a wave of hyperpolarization during the antagonist phase of locomotion. Experiments in the cat have shown that the depolarizing phase is due to an excitatory synaptic drive, while the hyperpolarizing phase is due to an inhibitory drive.
Plateau potentials are a behavior of the membrane potential in which the potential jumps between two states (bistable behavior). This motor neuron behavior has been observed in animals such as the turtle, mouse, and cat. This behavior is mediated mostly by a persistent inward current that at least in part is mediated by calcium channels. Ionic currents responsible for the generation of plateau potentials in motor neurons are found predominantly in dendrites because they possess voltage-sensitive L-type calcium conductances. These currents are turned ON by some neuromodulators such as serotonin or noradrenalin. Data from animal experiments have shown that synaptic inputs on dendrites can recruit plateau potentials and evoke sustained discharge. It is possible therefore that dendritic activation can amplify the effect of synaptic inputs from either sensory or descending afferents. Although the role of plateau potentials is still unclear during normal development, one hypothesis is that motor tasks like posture or stepping recruit fatigue-resistant motor units readily with sustained discharge produced by persistent inward currents without continuous network inputs.
From animal experiments there are some preliminary data that indicate that plateau potentials and sustained discharge are also found in some spinal interneurons. As in motor neurons, these plateaus are facilitated by serotonin and noradrenalin. In a similar fashion as in motor neurons, plateau potentials activated in interneurons by neuromodulators released during locomotion may act as an amplification step of synaptic inputs in particular dendrites. Because interneurons integrate sensory and supraspinal information, this mechanism may be very effective in the transmission of inputs relevant for the generation of motor neuronal activities.
George Mentis
Although these interneurons have been proposed as candidates forming the rhythm generating circuitry of the CPG, it is important to note that more work needs to be done to prove their essential role in rhythmogenesis. Not all rhythmically active neurons are components of the CPG network. Some may receive rhythmic output from CPG neurons. Causality needs to be established experimentally by temporarily and reversibly removing the participation of a population of interneurons during fictive locomotion. Strategies using genetic markers that identify specific populations of interneurons to drive selective expression of reversible inhibitors of activity provide a potential tool to test the contribution of distinct interneuron populations to CPG networks (Lechner, Lein, & Callaway, 2002).
Reciprocal Inhibition between Flexor and Extensor Muscles
Alternation between flexor and extensor muscles that act across the same joint is another fundamental feature of locomotion, as well as of many other limb movements. There are several spinal interneuron circuits that act in different ways to produce reciprocal inhibition between antagonist muscles. Presynaptic inhibition of antagonist Ia afferents by GABAergic interneurons, described earlier, prevents spindle afferents from activating a muscle when it is stretched by the contraction of its antagonist. Another interneuron, the glycinergic Ia inhibitory interneuron (IaIN), produces postsynaptic reciprocal inhibition. The IaIN was so named because it is activated by the muscle’s Ia afferents and inhibits motor neurons innervating the antagonist muscle (Wang, Li, Goulding, & Frank, 2008). The IaIN synapses on the soma and main dendrites of motor neurons, producing a fast, potent IPSP that lasts only for several milliseconds. The IaIN develops from the V1 cell lineage, and can be recognized by its expression of the transcription factor En1 (Siembab et al., 2010). Almost every input that excites a motor neuron will also excite the IaINs that project to its antagonist’s motor neurons. For this reason, voluntary contraction of a muscle will produce inhibition of its antagonist muscle, and, likewise, the stretch reflex of a muscle will produce inhibition of its antagonist. The excitability of IaINs is determined by their many inputs, and the strength of reciprocal inhibition can be varied to allow movements that use co-contraction. IaINs are rhythmically active during locomotion, even without feedback from muscle stretch (Pratt & Jordan, 1987). This observation led to the proposal that IaINs were involved in producing the flexor-extensor alternation of the locomotor CPG. However, this proposal is not supported by more recent studies using neonatal mice in which the V1 lineage neurons were reversibly inactivated. In this study, the locomotor rhythms became slower, but still exhibited alternation of flexors and extensors (Gosgnach et al., 2006).
Interneurons Involved in Left–Right Coordination
Interlimb coordination is the third fundamental feature of locomotion. To date, the leading candidate interneurons for coordinating activity between the limbs during locomotion are several populations of commissural interneurons (Fig. 28.10). Commissural interneurons have axons that cross the midline of the spinal cord at the level of the ventral commissure. They can be subdivided into three classes based on the direction of their axon: descending, ascending, or both (bifurcated axon). Descending commissural interneurons have been extensively studied in the rodent spinal cord during early development. Their axons project at least two spinal segments away from their soma to make contacts with other ventral horn interneurons and motor neurons. Some ascending commissural interneurons are cholinergic and are thought to transmit information to the upper parts of the spinal cord. Many of the commissural interneurons arise from the V0 and V3 cell lineage. The V0 lineage gives rise to ascending commissural interneurons, whereas the V3 lineage comprises a mixed population of commissural as well as ipsilaterally projecting neurons. In transgenic mice lacking the V0 commissural interneurons, the coordination between left and right legs was disrupted during locomotor rhythms, with episodic synchronous activation between sides (Lanuza, Gosgnach, Pierani, Jessell, & Goulding, 2004). These results suggest that this class of ascending commissural interneurons contributes, in part, to side-to-side interlimb coordination.
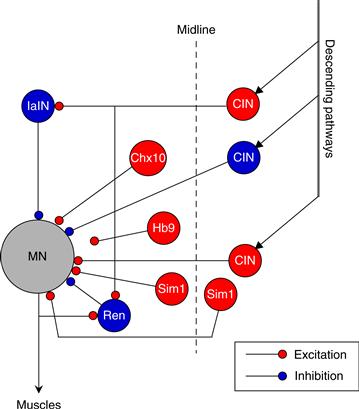
Figure 28.10 Schematic model with some of the known candidate interneurons that are involved in the modulation of the mammalian locomotor CPG. Excitatory interneurons (red) with rhythmic activity include classes of commissural interneurons (CIN) and the interneurons identified by expression of Sim1, Chx10, and Hb9 transcription factors. Inhibitory interneurons (blue) that participate in termination of excitatory bursts include the Renshaw (Ren), the Ia interneuron (IaIN), and some commissural interneurons (CIN).
Modified from Grillner (2006).
Recurrent (Renshaw) Inhibition
The recurrent collaterals of motor neurons synapse on a population of inhibitory interneurons in the ventral horn, called Renshaw cells (Renshaw, 1946). Renshaw cells use glycine or GABA as their transmitter and during development may contain both transmitters. Renshaw cells project back to homonymous motor neurons and synergist motor neurons in adjacent segments. They provide a negative feedback reflex called recurrent inhibition that limits the firing of motor neurons (Eccles, Fatt, & Koketsu, 1954). During locomotor rhythms, Renshaw cells are rhythmically active, as expected, since they receive rhythmic synaptic inputs from motor neurons. Their role in voluntary movements is uncertain, but one possibility is that they enhance the contrast between the active motor neurons and quiescent synergist motor neurons in adjacent segments. Renshaw cells arise from the V1 cell lineage, as do IaINs. During development, Renshaw cells, like IaINs, receive input from Ia afferents (Mentis, Siembab, Zerda, O’donova, & Alvarez, 2006). In the studies in neonatal mice, mentioned above, reversible inactivation of the V1 lineage neurons that produced slowing of locomotor rhythms included Renshaw cells and IaINs (Gosgnach et al., 2006).
Summary
In addition to serving as relays for sensory and descending pathways, interneurons of the spinal cord interconnect to form CPG networks that can produce rhythmic firing. Several models of the CPG network have been proposed to guide experimenters. There has been significant progress in unraveling the interneuronal network that forms the spinal central pattern generator for locomotion. New experimental techniques have allowed the identification in neonatal rodents of interneurons that are candidates for mediating some of the fundamental properties of locomotion: rhythmicity, alternation of antagonist muscles, and interlimb coordination.
Descending Control of Spinal Circuits
Neural circuits in the brainstem, cerebellum, and cerebral cortex are activated with locomotion and other movements. For locomotion, the cerebral cortex not only formulates conscious decisions about initiating and maintaining walking, but plays a role in adaptations of gait, particularly visually guided walking. Recordings from corticospinal neurons in cats as they stepped over obstacles placed in their field of vision showed that many corticospinal neurons changed their firing rates, often with a timing appropriate for driving the altered contraction patterns of specific muscles (Drew, Prentice, & Schepens, 2004). Corticospinal input, affecting motor neurons directly or through spinal CPG interneurons, can alter the limb’s trajectory with a high degree of precision, in a similar manner to a goal-directed reaching movement. In people, clinical observations and experimental studies suggest that supraspinal regions play a more active role in locomotor control than in lower mammals (Box 28.5).
Box 28.5 Neural Control of Human Walking
Human walking is a complex behavior that is based on integration of activity from descending supraspinal motor commands, spinal neuronal circuitries, and sensory feedback. Accumulating evidence suggests that humans, as well as other species, have a network in the spinal cord that is capable of generating basic rhythmic walking activity. Rhythmic leg movements can be induced by epidural electrical stimulation after a clinically complete spinal cord injury (SCI). Spontaneous involuntary rhythmic leg movements have also been reported after a clinically complete and incomplete SCI. However, even though this evidence suggests the existence of a CPG network in humans, in all these cases sensory input likely contributed to rhythm generation. Furthermore, clinically complete injuries are not always anatomically complete. As a result, the evidence for existence of a CPG in humans remains indirect.
Noninvasive electrophysiological techniques have provided information about how certain brain structures contribute to human walking. For example, the involvement of the primary motor cortex, where the corticospinal tract originates, has been demonstrated, in part, with transcranial magnetic stimulation (TMS). Several groups have found changes in the size of motor evoked potentials (MEP) in lower limb muscles elicited by TMS during walking, showing that transmission in the corticospinal tract is modulated during the gait cycle. However, TMS of the motor cortex activates cells with monosynaptic or polysynaptic connections to the spinal motor neurons. The size of the MEPs elicited by TMS reflects not only cortical excitability changes, but also changes at a sub-cortical level. To more precisely identify the various sites activated by the magnetic stimulus, Petersen, Christensen and Nielson (1998) tested the effect of TMS on the H-reflex, an electrical homologue of the monosynaptic stretch reflex, during walking. They found that at least part of the MEP modulation was caused by changes in the excitability of corticospinal cells with direct monosynaptic projections to the spinal motor neurons. They also found that TMS over the motor cortex at intensities below the threshold for activating spinal motor neurons depressed ongoing muscle activity in the tibialis anterior muscle of the leg during walking. This effect was thought to be caused by activation of cortical inhibitory interneurons targeting cortical pyramidal tract neurons (Petersen et al., 2001).
During human walking, sensory feedback plays an important role in driving the active motor neurons and providing error signals to the brain that can be used to adapt and update the gait pattern. In healthy humans, a study in which a plantarflexion perturbation was induced during the stance phase of the gait cycle provides clear evidence for this. This experiment showed a drop in electromyographic (EMG) activity in the plantarflexor muscles, even when the common peroneal nerve that innervates the ankle dorsiflexor muscles was blocked by local anesthesia (Sinkjaer, Anderson, Ladouceur, Christenson, & Nielson, 2000). These results demonstrated that sensory feedback from plantarflexor muscle contributed to the active drive of motor neurons during walking. Additional information about the contribution of sensory feedback to the control of locomotion has been gathered from studies that investigate changes in EMG responses in lower limb muscles after stimulation of cutaneous and proprioceptive afferents during walking. The amplitudes of the EMG responses to electrical stimulation are dependent on the phase (or time) of stimulation during the gait cycle. For example, electrical stimulation of the human sural nerve results in facilitation of the ankle flexor muscle tibialis anterior during early swing, but leads to suppression when delivered during late swing (reflex reversal). It has been proposed that the phase-dependent modulation of EMG responses may have functional relevance at various times in the step cycle. For example, a flexor reflex is appropriate at the onset of the swing phase when the leg is flexed, but the same reflex is not convenient at the end of the swing phase when the foot is ready to take up body weight. To some extent this modulation is mediated by supraspinal centers. To examine this possibility, a combination of cutaneous input and activation of supraspinal pathways by TMS has been used during the gait cycle. Available data suggest that long-latency reflexes elicited by cutaneous electrical stimulation are in part transcortically meditated.
In summary, in recent years a combination of neurophysiological techniques has provided important insights that have improved our understanding about the neural control of human walking. This behavior is based on the integration of activities from spinal circuitry, sensory feedback, and descending motor commands.
Monica Perez
Brainstem Control of Spinal Interneuron Networks
The brain transmits signals for movement to the spinal cord through several descending fiber tracts: corticospinal, vestibulospinal, and reticulospinal, as described in the following chapter. For locomotion, supraspinal regions are thought to activate spinal cord CPGs through brainstem relays. In decerebrate cats, electrical stimulation of several areas of the brain can elicit stepping. These include the diencephalon and cerebellum, but the most critical region is an area in the brainstem near the pedunculopontine nucleus known as the mesencephalic locomotor region (MLR). The MLR receives inputs from several forebrain regions, including the basal ganglia. The MLR does not connect directly to spinal CPG neurons, but synapses on reticulospinal neurons in the pons and medulla that receive a wide variety of sensory and descending inputs. Some of the reticulospinal axons in the pontomedullary formation travel in the ventral and ventrolateral tracts (funiculi) of the spinal cord. Lesions of these funiculi disrupt the ability to initiate and maintain locomotion. Sparing of at least some fibers in the ventrolateral funiculus markedly improves locomotor recovery after spinal lesions in cats (Rossignol, Drew, Brustein, & Jaing, 1999) and could be critical for the recovery of locomotor movements after spinal injury in humans.
In addition to their role in triggering rapid changes in locomotion and regulating postural balance, descending fiber systems also modulate physiological properties of spinal neurons. For instance, the plateau potentials and bistability in motor neurons (Box 28.4) depend on tonic activity in descending serotonergic pathways. Stimulation of the dorsolateral funiculus of the spinal cord in turtles, an area which is thought to contain descending pathways from the raphe nucleus, increased the excitability of motor neurons and promoted production of plateau potentials.
Sensory Modulation
Although spinal CPGs can generate the basic pattern of neural activity that organizes muscle contractions into a recognizable movement such as locomotion, the final pattern is fine-tuned by sensory inputs as well as descending systems. As noted earlier, most sensory inputs affect motor neurons through polysynaptic, interneuronal circuits. The interneurons that process sensory information typically integrate sensory signals with inputs from a variety of sources, including CPG networks and descending pathways. As a result the reflex motor response can vary depending on the phase of the movement cycle, or it may only occur when the animal is in the “state” of moving, or it may even be reversed between resting and active states. Earlier we discussed the muscle sensory receptors and some of the reflex changes they mediate. This last section will present a sampling of other sensory reflex pathways.
Flexor Reflexes
A wide variety of sensory stimuli can evoke reflex flexion of the limb. Flexor reflexes may have several roles: they serve a protective function, reflexively withdrawing the limb from potentially harmful stimuli, and they may be used in movements that require coordinated multijoint flexor movements. Usually the reflex consists of contraction of muscles that flex several joints coupled with relaxation of muscles that extend those joints. Extension of the contralateral limb may also occur. Flexor reflexes can be elicited by group II and III muscle afferents, joint afferents, and high- and low-threshold cutaneous afferents. Because these afferents elicit a common action, they are sometimes called “flexor reflex afferents” (FRAs). The muscles activated by the sensory stimulus respond with short and long latency bursts of contraction. These contraction components are thought to be generated by distinct interneuron circuits. The late components can be differentially enhanced by treatment with L-dopa in spinalized animals. It has been suggested that interneurons mediating the late flexor reflex could function as a half-center for flexion, as proposed in models of the locomotor CPG.
Cutaneous Reflexes
In contrast to the widely distributed flexor reflex, there are a number of reflexes in which stimulation of small areas of skin evokes a reflex limited to a few muscles. One example of a local cutaneous reflex is the stumbling corrective reaction. When the skin on the top of the foot is stimulated during walking, the foot is lifted higher, as if to step over an obstacle. The stumbling corrective reaction is phase dependent, occurring only when the limb is in the swing phase of the step cycle. This and several other local cutaneous reflexes are oligosynaptic, involving only a few interneurons. The last-order interneurons in these cutaneous reflexes connect with relatively limited sets of motor neurons. It has been proposed that such interneurons offer a means to control or alter activity of specific muscles during movement without involving the interneurons of pattern generating circuitry.
Summary
Several areas of the brain give rise to descending pathways that connect to spinal interneurons and, to varying extents in different species, to motor neurons. Areas of the brainstem are critical for relaying descending commands for locomotion. Volitional movements also seem to employ spinal interneuron circuits for organizing routine patterns of muscle contraction, within the context of a planned goal. Sensory feedback from skin, joints, and most muscle afferents provide fine-tuning of basic movement patterns. The reflex motor responses evoked by most sensory afferents are relayed through interneurons.
References
1. Alaynick WA, Jessell TM, Pfaff SL. SnapShot: Spinal cord development. Cell. 2011;146 178.
2. Alvarez FJ, Fyffe RE. The continuing case for the Renshaw cell. Journal of Physiology. 2007;584:31–45.
3. Betley JN, Wright CV, Kawaguchi Y, et al. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell. 2009;139:161–174.
4. Boillee S, Vande Velde C, Cleveland DW. ALS: A disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59.
5. Bonnot A, Whelan PJ, Mentis GZ, O’Donovan MJ. Locomotor-like activity generated by the neonatal mouse spinal cord. Brain Research Reviews. 2002;40:141–151.
6. Brown TG. The intrinsic factors in the act of progression in the mammal. Proceedings of the Royal Society of London. 1911;84:308–319.
7. Burke RE, Levine DN, Tsairis P, Zajac FE. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. Journal of Physiology (London). 1973;234:723–748.
8. Butt SJ, Lundfald L, Kiehn O. EphA4 defines a class of excitatory locomotor-related interneurons. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14098–14103.
9. Cooper EC, Jan LY. Ion channel genes and human neurological diseases: Recent progress, prospects, and challenges. Proceedings of the National Academy of Sciences of the USA. 1999;96:4759–4766.
10. Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. Journal of Neurophysiology. 1997;77:247–259.
11. Crone SA, Quinlan KA, Zagoraiou L, et al. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron. 2008;60:70–83.
12. Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Progress in Brain Research. 2004;143:251–261.
13. Eccles JC, Fatt P, Koketsu K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. Journal of Physiology. 1954;126:524–562.
14. Gosgnach S, Lanuza GM, Butt SJ, et al. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440:215–219.
15. Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nature Reviews Neurosciences. 2009;10:507–518.
16. Goulding M, Pfaff SL. Development of circuits that generate simple rhythmic behaviors in vertebrates. Current Opinion in Neurobiology. 2005;15:4–20.
17. Grillner S. Neurobiological bases of rhythmic motor acts in vertebrates. Science. 1985;228:143–149.
18. Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766.
19. Grillner S, Jessell TM. Measured motion: Searching for simplicity in spinal locomotor networks. Current Opinion in Neurobiology. 2009;19:572–586.
20. Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1347.
21. Hinckley CA, Hartley R, Wu L, Todd A, Ziskind-Conhaim L. Locomotor-like rhythms in a genetically distinct cluster of interneurons in the mammalian spinal cord. Journal of Neurophysiology. 2005;93:1439–1449.
22. Jessell TM. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nature Review Genetics. 2000;1:20–29.
23. Kullander K, Butt SJ, Lebret JM, et al. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science. 2003;299:1889–1892.
24. Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron. 2004;42:375–386.
25. Lechner HA, Lein ES, Callaway EM. A genetic method for selective and quickly reversible silencing of Mammalian neurons. Journal of Neurosciences. 2002;22:5287–5290.
26. Mentis GZ, Alvarez FJ, Bonnot A, et al. Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proceedings National Academy Sciences USA. 2005;102:7344–7349.
27. Mentis GZ, Siembab VC, Zerda R, O’donovan MJ, Alvarez FJ. Primary afferent synapses on developing and adult Renshaw cells. Journal of Neurosciences. 2006;26:13297–13310.
28. Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O. Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proceedings of National Academy of Sciences USA. 2005;102:5245–5249.
29. Petersen NT, Butler JE, Marchand-Pauvert V, et al. Suppression of EMG activity by transcranial magnetic stimulation in human subjects during walking. Journal of Physiology (London). 2001;537:651–656.
30. Petersen N, Christensen LO, Nielsen J. The effect of transcranial magnetic stimulation on the soleus H reflex during human walking. Journal of Physiology (London). 1998;513:599–610.
31. Pratt CA, Jordan LM. Ia inhibitory interneurons and Renshaw cells as contributors to the spinal mechanisms of fictive locomotion. Journal of Neurophysiology. 1987;57:56–71.
32. Renshaw B. Central effects of centripetal impulses in axons of spinal central roots. Journal of Neurophysiology. 1946;9:191–204.
33. Rossignol S, Drew T, Brustein E, Jaing W. Locomotor performance and adaptation after partial or complete spinal cord lesions in the cat. Progress in Brain Research. 1999;123:349–365.
34. Rudomin P. In search of lost presynaptic inhibition. Experimental Brain Research. 2009;196:139–151.
35. Siembab VC, Smith CA, Zagoraiou L, Berrocal MC, Mentis GZ, Alvarez FJ. Target selection of proprioceptive and motor axon synapses on neonatal V1-derived Ia inhibitory interneurons and Renshaw cells. Journal of Comparative Neurology. 2010;518:4675–4701.
36. Sinkjaer T, Andersen JB, Ladouceur M, Christensen LO, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. Journal of Physiology (London). 2000;523:817–827.
37. Tresch MC, Kiehn O. Coding of locomotor phase in populations of neurons in rostral and caudal segments of the neonatal rat lumbar spinal cord. Journal of Neurophysiology. 1999;82:3563–3574.
38. Wang Z, Li L, Goulding M, Frank E. Early postnatal development of reciprocal Ia inhibition in the murine spinal cord. Journal of Neurophysiology. 2008;100:185–196.
39. Wilson JM, Hartley R, Maxwell DJ, et al. Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. Journal of Neurosciences. 2005;25:5710–5719.
40. Zhang Y, Narayan S, Geiman E, et al. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron. 2008;60:84–96.
41. Zhong G, Droho S, Crone SA, et al. Electrophysiological characterization of V2a interneurons and their locomotor-related activity in the neonatal mouse spinal cord. Journal of Neuroscience. 2010;30:170–182.
Suggested Readings
1. Engel AG, Sine SM. Current understanding of congenital myasthenic syndromes. Current Opinion in Pharmacology. 2005;5:308–321.
2. Kiehn O. Locomotor circuits in the mammalian spinal cord. Annual Review Neurosciences. 2006;29:279–306.
3. O’Donovan MJ, Bonnot A, Wenner P, Mentis GZ. Calcium imaging of network function in the developing spinal cord. Cell Calcium. 2005;37:443–450.
4. Schwab JM, Brechtel K, Mueller CA, et al. Experimental strategies to promote spinal cord regeneration—an integrative perspective. Progress in Neurobiology. 2006;78:91–116.