Chapter 42
Human Brain Evolution
Human Brain Evolution
Modern evidence concerning brain evolution from comparative studies shows that brain structure, far from being uniform across species, exhibits remarkable variations. This chapter focuses on the evolutionary history of three groups of vertebrates that are of special interest to people: mammals, primates, and humans themselves. The major steps are outlined in the evolution of our large, complex, and extremely useful brains from the smaller, simpler brains of the first mammals, with an emphasis on the neocortex, since this part of the brain is disproportionately large in humans and is critically involved in mental activities and processes that are considered to be distinctly human. We review new evidence about the changes in the internal organization of brains that took place after the human lineage diverged from the lineage leading to our closest relatives, chimpanzees.
The Brains of Early Mammals
As early as 340 mya, early reptiles, now most often called “stem amniotes,” evolved from amphibians and diverged into two major clades, the sauropsids, which led to present-day reptiles and birds, and the synapsids, which left no survivors other than mammals (Fig. 42.1). We know from the fossil record that early mammals were mainly small, mouse- to cat-sized, and the endocasts of their skulls (Box 42.1) indicate that they had small brains in proportion to their body sizes. In addition, their forebrains were dominated by a large olfactory bulb and a proportionately large expanse of olfactory cortex. In contrast, neocortex was restricted to a small cap on the dorsal surface of the forebrain that failed to extend over the midbrain, as it does in most extant mammals. Thus, olfaction was a very important sense in early mammals, and neocortex was not the dominant structure that it is in most extant mammals. In addition, most early mammals appeared to have been nocturnal with rather poor vision. However, early mammals evolved small bones in the middle ear, the malleus and incus, which together with the stapes transmit oscillations of air pressure to the cochlea of the inner ear. This, and modifications of the cochlea, allowed early mammals to extend their hearing into high frequencies, permitting communication signals that would be undetected by reptilian predators (Allman, 1999). As auditory processing became more important, the inferior colliculus of the auditory midbrain became larger than its visual counterpart, the superior colliculus (Fig. 42.2). As with many extant mammals, touch receptors for facial vibrissae and for the tongue and lips probably provided much of the somatosensory input.
Box 42.1 How Do We Learn About Brain Evolution?
There are three main ways to learn about how different brains have evolved. First, the fossil record can be studied. Because bones readily fossilize, whereas soft tissues seldom do, we know a lot about the bones of our ancestors, but much less about everything else. Of course one can infer much about some soft tissues, such as muscles, from their effects on bones, and this is true for brains as well. The brains of mammals fill the skull tightly, and thus the skull cavity of fossils (the endocasts) rather closely reflects the size and shape of the brain, and even the locations of major fissures. Much has been learned about the changes in brain size from the fossil record (Jerison, 2007), and we learn more by considering changes in the proportions of brain parts. For example, early primates already differ from most early mammals by having more neocortex in proportion to the rest of the brain, and more neocortex devoted to the temporal lobe where visual processing occurs. This implies a greater emphasis on functions mediated by neocortex and a greater emphasis especially on the processing of visual information.
We can even learn something about the functional organization of the neocortex from fossils. For instance, subdivisions of the body representation in the primary somatosensory cortex are often marked by fissures in the brain, and fissure patterns revealed by endocasts from fossil skulls have been used to suggest specializations of the somatosensory cortex in extinct mammals (Radinsky, 1976). David Van Essen (2007) has proposed that an important factor in the development of brain fissures is the pattern of connections within the developing brain. According to this theory, densely interconnected regions tend to resist separation during brain growth and form bulges (gyri) that limit the separation distance, whereas poorly interconnected regions are free to fold and form fissures (sulci) that would increase the separation distance. Thus, it is because the hand and face regions of the somatosensory cortex are poorly interconnected that a fissure may develop between the two. In a similar manner, the position of the lunate sulcus has been used to indicate the junction of primary with secondary visual cortex in fossil endocasts of hominid skulls (de Sousa et al., 2010). Thus, the locations of brain fissures in fossil endocasts may tell us something about anatomical connections and functional boundaries in the brains of extinct mammals.
Most of what is known about the evolution of brains is based on comparisons of brain organization of different species of present-day (extant) mammals and other vertebrates. As Darwin recognized, each living species represents the living tip of a largely dead branch of an extremely bushy tree of life. By examining other living tips of the tree, we can infer much about the organizations of the brains (and other body parts) of ancestors that occupied the branching points of this tree. Theories of brain evolution, including those of the evolution of the human brain, depend on reconstructing the probable features of the brains of ever more distant relatives. The comparative method depends on (1) examining the brains of suitable ranges of extant species and (2) determining what features they share, and whether these features are shared because they were inherited from a common ancestor or because they evolved separately. The field of cladistics provides guidelines for making such judgments. The choice of species for comparison depends on the question being asked. For example, to deduce what the brains of early mammals were like, one should examine brains from each of the major branches of the mammalian evolutionary tree (Fig. 42.1). In order to learn about early primates, we need to consider the major branches of primate evolution along with mammals that are thought to be closely related to primates such as tree shrews.
Although the brains of some mammals may have retained more primitive characteristics than others, it is unlikely that the brain of any living mammal fully represents an ancestral condition. Brain features need to be evaluated trait by trait in a comparative context, as any particular feature could be primitive (ancestral) or derived (modified). To reconstruct the course of brain evolution, we need to distinguish ancestral and derived characters. We expect modern species to exhibit a mixture of features that have been retained from a range of ancestors from recent-to-ancient, as many features or traits are thought to evolve independently (called mosaic evolution), although features can be linked and evolve together.
A third source of information about brain evolution is based on understanding the mechanisms and modes of brain development and the constraints they impose on evolution. For example, Finlay and Darlington (1995) have presented evidence that brains change in orderly ways, as they get bigger. In general, larger brains have proportionately more neocortex and less brain stem. Finlay and Darlington suggest this is the case because the late-maturing neocortex of large brains grows longer (late makes great). Another difference between large and small brains is that large brains have more neurons and longer connections. The increase in neurons makes it difficult for each neuron in a large brain to maintain the same proportion of connections with other neurons, as do neurons in a small brain, and to maintain the same transmission times over longer axons. Thus, as larger brains evolve, changes in organization are needed to reduce the commitment to connections, especially connections requiring long, thick axons. A deeper understanding of the genetic, developmental, and structural constraints on brain design could allow us to better postulate how brains are likely to change in organization with changes in brain size.
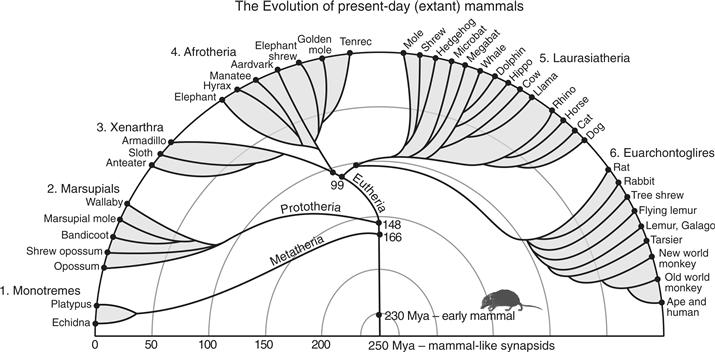
Figure 42.1 The phyletic radiation of extant mammals into six major clades or superorders. Most early mammals were shrew-like in appearance. They emerged from mammal-like synapsids about 230 million years ago (mya). Humans and other primates are in the Euarchontoglire superclade. Based on Murphy, Pevzner, and O’Brien (2004).
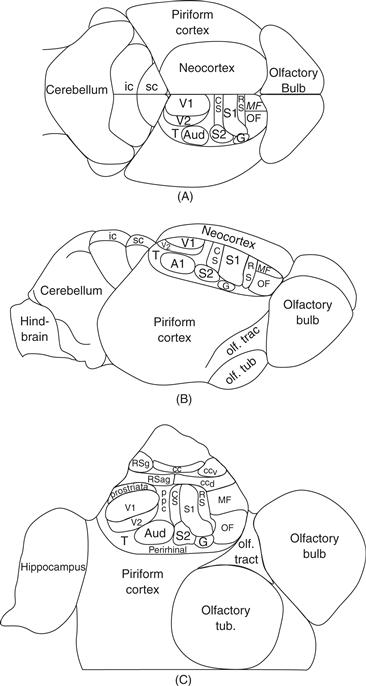
Figure 42.2 The inferred organization of neocortex in early mammals. The figure emphasizes the small neocortex in proportion to olfactory piriform cortex and olfactory bulb reflected in the brain endocasts of early mammals. Comparative studies of extant mammals reveal cortical areas that are widespread across mammalian clades, suggesting that they have been retained from early mammalian ancestors. A. Dorsal view of the brain with cortical areas indicated. B. Lateral view. C. View of cortex after it has been separated from the brainstem and unfolded to reveal cortex of the medial wall of the cerebral hemisphere. The hippocampus has been unfolded. Cortical areas include primary and secondary visual areas, V1 and V2, primary and secondary somatosensory areas, S1 and S2, a primary-like auditory core, Aud., caudal and rostral somatosensory belts bordering S1, CS and RS, a gustatory area, G, orbital frontal, OF, and medial frontal, MF, cortex, posterior parietal cortex, PPC, temporal visual cortex, T, dorsal and ventral cingulate cortex, CCd and CCv, and agranular and granular retrosplenial cortex, RSag and RSg, corpus callosum, CC, inferior colliculus, ic, superior colliculus, sc, olfactory tubercle, Olf. Tub., and olfactory tract, Olf. Trac.
The neocortex of early mammals was perhaps the part of the brain most changed from early ancestors. Neocortex is not, as its name implies, a new part of the brain, as neocortex is homologous to the dorsal cortex of reptiles. Thus, a forebrain structure in early amniotes evolved into neocortex in the sauropsid line leading to mammals and to dorsal cortex in the line leading to modern reptiles and birds (the hyperpallium or wulst in birds). Although it is not known exactly how the dorsal cortex of early amniotes evolved into the neocortex of early mammals, the dorsal cortex of the sauropsid ancestors of mammals is generally thought to resemble the dorsal cortex of present-day reptiles, such as turtles (Fig. 42.3). The dorsal cortex of reptiles consists of a single layer of pyramidal neurons capped by a layer of input axons mixed with dendrites of the pyramidal neurons and a few (10–20 percent) largely inhibitory intrinsic neurons. The fiber layer under the pyramidal cells consists of the axons leaving the pyramidal cells. This type of laminar and cellular organization is also found in medial cortex of reptiles that is homologous to the hippocampal formation of mammals and birds, and the lateral or piriform olfactory cortex of mammals, reptiles, and birds (Shepherd, 2011). However, dorsal cortex of mammals evolved new cell layers, to become the familiar six-layered neocortex of mammals. The single layer of pyramidal cells of dorsal cortex was expanded to form the superficial layers 2 and 3, and the deep layers 5 and 6, with each layer specialized for receiving different inputs and providing different outputs, while a middle layer 4 of stellate cells was specialized for receiving fine-grain sensory or other information from the thalamus or other areas of cortex and distributing it to the other layers (Shepherd, 2011). In addition to having the potential to form new kinds of local circuits, the neocortex of early mammals had the potential to be subdivided into any number of functionally different cortical areas that could relate to other areas in various ways, and these cortical areas could be subdivided into sets of repeating modules or columns, providing further computational advantages. As a result, neocortex became a very important part of the brain in some mammals, providing 80 percent of the total brain mass in humans (Azevedo et al., 2009). However, large brains with massive amounts of neocortex came with great costs, including considerable metabolic requirements, and long development times. Larger brains also generate more heat, and are difficult to keep cool (Falk, 1990). In order to have larger amounts of neocortex, a mammal needs to have the potential of a relatively long life and access to high-quality food (Aiello & Wheeler, 1995). Thus, many mammals have small brains and little neocortex with limited computational ability, and likely short lives that are compensated by early maturation, lower metabolic costs, and early reproduction.
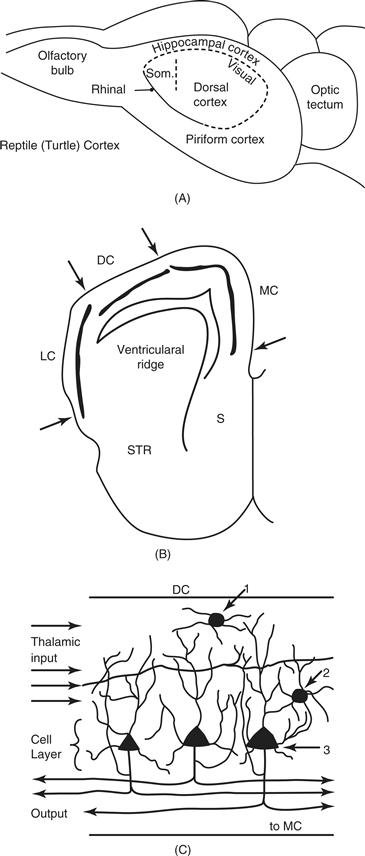
Figure 42.3 Dorsal cortex of reptiles. A. A dorsolateral view of a turtle brain. The large olfactory bulb provides input to lateral cortex (homologue to piriform cortex of mammals). A slight rhinal dimple is apparent rostrally at the border of dorsal cortex (homologous to neocortex of mammals). Medial or hippocampal cortex (homologous to the hippocampus of mammals) is medial. Most of dorsal cortex receives visual inputs, while a small rostral zone receives somatosensory (som.) inputs. The large optic tectum is part of the midbrain. B. A frontal section through the forebrain of a reptile showing the locations of the dorsal cortex (DC), the lateral cortex (LC) and the medial cortex (MC). The solid lines in these cortical areas represent the densely packed pyramidal neurons that form a single cell layer in all three areas. S = septum; STR = striatum. C. The cellular structure of dorsal cortex. A densely packed row of pyramidal neurons forms a middle layer. Pyramidal neurons constitute 80 to 90 percent of the neurons in DC. They receive inputs from the visual thalamus as thalamocortical axons stream from lateral to medial in dorsal cortex synapsing on many pyramidal neurons. A few subpial and stellate cells are inhibitory on pyramidal neurons after being activated by pyramidal neuron axons, and input axons. The main branch of pyramidal neuron axon bifurcates with one collateral coursing medially to MC, and the other collateral coursing to the striatum, the visual thalamus, and the optic tectum. Other branches activate other pyramidal neurons and intrinsic neurons. 1. Subpial interneuron. 2. Stellate interneuron. 3. Pyramidal neuron.
Although the neocortex of early mammals was small in comparison to the rest of the brain, it already had several functionally distinct subdivisions. By comparing the organization of neocortex in the brains of members of the six major clades (a clade is any group where all members descended from a common ancestor) of extant mammals (Fig. 42.1), especially those mammals with small brains and relatively little neocortex, we can identify features these brains have in common as a result of sharing an early mammal ancestor. Such a comparative approach leads to several well-supported conclusions (Kaas, 2007a, 2007b, 2011a). First, cortical areas were more similar to each other structurally, and less specialized, than in most extant mammals. While most areas of neocortex had the familiar pattern of six layers of cells, the layers were not markedly different in the types and shapes of intrinsic neurons and pyramidal neurons. Cortical areas were structurally similar, although primary sensory areas were probably already distinguished by having a layer 4 that was more densely packed with small neurons than other cortical areas, and retrosplenial cortex on the medial wall of the cerebral hemisphere was distinguished by having a thin, poorly differentiated layer 4 and a relatively thick layer 2. In general layer 2 was more distinct and layer 1 thicker across cortex than in most extant mammals, reflecting the retention of aspects of dorsal cortex. The structural appearance of neocortex in marsupial opossums (Wong & Kaas, 2009) roughly approximates that expected for early mammals.
We can also conclude that the small cap of neocortex of early mammals was subdivided into a small number of functionally distinct areas, on the order of 10–20, and that these areas have been retained in most lines of descent. From slight regional differences in the laminar appearance of neocortex, early investigators such as Brodmann (1909) surmised that all mammals have functionally significant subdivisions of cortex called areas. Some of these areas are shared by many species (homologous areas retained from a common ancestor), while others are found only in some branches of mammalian evolution. However, it was difficult for Brodmann and other early investigators to reliably identify areas by their differences in histological appearance, especially in the poorly differentiated cortex of most small-brained mammals, but also in the large expanses of rather homogeneous cortex present in large-brained mammals. As a result, areas were often incorrectly delimited within species, and misidentified across species. Fortunately, modern methods of investigation allow many features of cortical biology to be compared in great detail, including patterns of neural connections, ways of representing sensory and other inputs, neuron response properties, and cortical histochemistry. This added information allows cortical areas to be identified within and across species with much more assurance. Nevertheless, many areas remain difficult to identify and delimit with certainty.
When we consider the known distribution of cortical areas in small-brained mammals from the major branches of the mammalian radiation (Fig. 42.1), such as opossums, armadillos, tenrecs, hedgehogs, and even rats (Kaas, 2011a), we find a number of shared areas (Fig. 42.3). Except for a few blind or nearly blind species, such as mole-rats, mammals have a caudally located primary visual area, V1, that is architectonically distinct, has dense inputs from the lateral geniculate nucleus of the visual thalamus, and contains a representation of the contralateral visual hemifield. Other visual areas have been harder to define, but there is widespread evidence across many species for a second visual area, V2, along the lateral border of V1. Thus, it appears that early mammals had a V2, and this V2 has been retained in most present-day mammals. Exceptions are nearly blind mammals, and a few species such as the least shrew that evolved to have extremely small bodies and brains, resulting in the loss of V2 and a few other areas (Catania, Lyon, Mock, & Kaas, 1999). Evidence from comparative studies also supports the conclusion that early mammals also had a visual area along the medial border of V1, a less well-studied area given several names, but commonly called the prostriate area. In addition, one visual area or more was likely located lateral to V2 in temporal cortex of early mammals (T in Fig. 42.2). Because the dorsal cortex of reptiles is predominantly visual in function (Fig. 42.3), one or more visual areas likely existed in the dorsal cortex of the synapsid ancestors of mammals.
The organization of auditory cortex has been less studied across a range of mammalian taxa, but a primary or primary-like region has been identified in ventral temporal cortex of all studied mammals (Kaas, 2011b). In many of these mammals, the cortex with primary-like architectonic features includes two or sometimes three different representations of tone frequencies, with one of the representations termed the primary area, A1, and the other, the anterior auditory field among other names. Without further comparative studies, it is uncertain if the primary auditory region of early mammals (Aud) contained a single area, A1, or more. This auditory core area was likely bordered by one or more secondary (belt) auditory fields, but this remains uncertain. Auditory inputs apparently are not represented in dorsal cortex of reptiles and may not have been present in synapsids.
All mammals have several somatosensory areas. A primary area, S1, represents touch on the contralateral half of the body from the tail to the tongue in a mediolateral sequence across anterior parietal cortex. Lateral to S1, a second somatosensory area, S2, has been identified in a wide range of mammals, and the adjoining parietal ventral area, PV, has often been reported, suggesting that S2 and possibly PV (not shown in Fig. 42.2, but located just rostral to S2) were present in early mammals. In addition, narrow bands of cortex along the rostral and caudal borders of S1 with inputs from S1 likely existed, termed here the rostral and caudal somatosensory areas, RS and CS. RS likely had proprioceptive inputs as in area 3a of cats and primates and dysgranular somatosensory cortex of rats. Perhaps surprisingly, early mammals did not have a primary motor area, M1, or any of the premotor areas that characterize primates. This follows from the lack of compelling evidence for an M1 or premotor cortex in marsupials or monotremes, animals that represent early branches of the mammalian radiation. However, inputs from the cerebellum to the anterior thalamus were probably present, and this motor-related information was distributed to somatosensory cortex, which had motor as well as sensory functions. As dorsal cortex in reptiles appears to have a small region with somatosensory inputs, a region of somatosensory cortex may have been present in the synapsid ancestors of mammals. Finally, there may have been a small region of gustatory cortex, G, located lateral and somewhat rostral to S1 in ancestral mammals, but this is uncertain, as there have been few comparative studies of the representation of taste in the cortex of mammals.
Other cortical areas have been less widely identified in comparative studies, so their presence in early mammals is uncertain. Yet the architectonic features of the granular and agranular retrosplenial areas, RSg and RSa, are distinctive enough to have them be widely identified. There is also reasonably good evidence that early mammals had at least two divisions of cingulate cortex, and two divisions of frontal cortex. Overall, early mammals had a thick neocortex of six layers that was subdivided into as many as 15 or more cortical areas. How this neocortex emerged from the thin, small dorsal cortex with possibly only visual and somatosensory inputs in the ancestors of mammals remains a challenging question (Puelles, 2011).
From this ancestral pattern, a great variety of brain organizations have evolved through alterations in the sizes and numbers of parts, and the connections within and between parts (Kaas, 2007a). Consider, for example, the variations of the primary somatosensory cortex. The duck-billed platypus devotes most of S1 to representing tactile receptors of its highly sensitive bill, and it has added inputs from electroreceptors. The star-nosed mole devotes most of S1 to its long, fleshy nose appendages, rats mostly activate S1 with their facial whiskers, and human S1 has a large representation of the hand, lips, and mouth. In addition, the amount of neocortex varies greatly across species of mammals (Jerison, 2007), and some of this variation is due to differences in numbers of cortical areas present in different groups of mammals (Changizi & Shimojo, 2004; Finlay & Brodsky, 2007; Kaas, 2007a). The number of sensory areas increased independently in several lines of evolution. For example, both cats and monkeys have a large number of visual areas, but the carnivore and primate lines appear to have acquired most of these areas independently rather than from a common ancestor. Thus, many of the visual areas in cats have no homologs in monkeys or humans. A similar situation holds for other regions of the brain. New areas were added, most commonly to sensory and motor regions of cortex rather than to multisensory “association” cortex as once thought. However, sensory areas typically are modulated by other inputs including other sensory and nonsensory inputs. With new cortical areas, new connections between areas and subcortical structures emerged. How new areas evolve remains unclear: they could result from the duplication of existing areas, or by the gradual subdivision of existing areas, or both.
Thus, mammals in various lines of descent evolved new cortical areas and connections, as well as many other specializations of previously existing areas. A long-recognized example of a new brain feature is the corpus callosum, a derived character of placental mammals, having emerged in the ancestors of placentals after they diverged from marsupials. Whereas connections between the two hemispheres are mediated by the anterior commissure in marsupials and monotremes, most of these connections are carried in the shorter, more direct callosal pathway in placental mammals. Primary motor cortex, M1, is another important brain feature that appears to have emerged in early placental mammals after the split from marsupials.
Evolution of Primate Brains
Evolution of Primates
Early primates emerged from small-brained, nocturnal, insect-eating mammals, perhaps as much as 80 million years ago (Martin, 2007), and subsequently branched into two main lineages (Fig. 42.4). One lineage, the strepsirrhines, includes lemurs, lorises, and galagos (collectively, most of the “prosimian” primates). A second lineage, the haplorhines, includes two important daughter branches, one leading to the small, strepsirrhine-like tarsiers, the other leading to the familiar anthropoid primates, which include a New World radiation (the platyrrhines, or New World monkeys) and an Old World radiation (the catarrhines, comprising the Old World monkeys and the ape-human group).
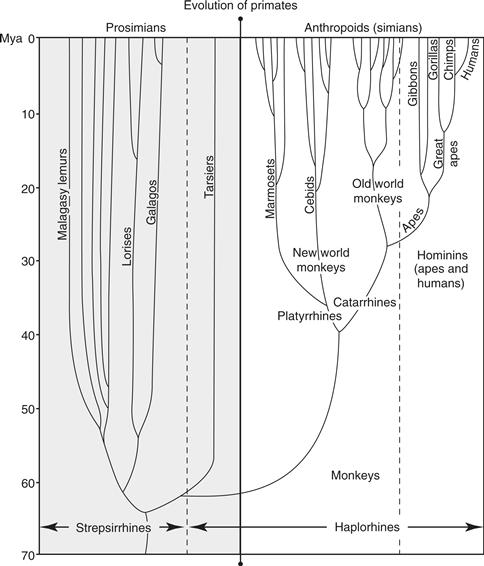
Figure 42.4 The evolution and classification of primates. Tarsiers are generally considered to be prosimians, but they are related more closely to anthropoids, so they are recognized as haplorhine primates. Despite the ancient split of strepsirrhines and haplorhines, they share many brain features that are unique to primates. Tree shrews and flying lemurs are thought to be close relatives of primates, and together with them constitute the superorder Archonta. See text.
The earliest primates are thought to have been small-bodied, nocturnal visual predators living on insects and small vertebrates, as well as fruit. They adapted to the tropical rainforests by emphasizing vision and visually guided reaching and grasping. This involved having larger, forward-facing eyes, opposable big toes and thumbs, and digits tipped with nails rather than claws. These primates produced the largely nocturnal strepsirrhine radiation with its varied forms, including some species now living in Madagascar that have become diurnal. The haplorhine primates emerged about 60 million years ago in association with a shift from nocturnal to diurnal life, together with an increased emphasis on fruit eating (Ross, 1996). With the shift to diurnal activity came reduced dependence on olfaction, enhancement of the visual system, enlarged body size, and sometimes a more gregarious mode of life. Specifically, the olfactory apparatus was reduced in size, and the eyes enlarged and brought close together. Early haplorhines evolved a fovea, a specialized region of the retina filled with small photoreceptors and devoid of blood vessels that enhances central visual acuity. The reflecting surface at the back of the eye (tapetum lucidum), an adaptation to nocturnal vision present in strepsirrhines and many other nocturnal mammals, was lost. Anthropoids underwent further specializations, modifying cone morphology, increasing the proportion of cones to rods, and filling the fovea with cones to the near exclusion of rods. These adaptations enabled anthropoids to achieve a high degree of color discrimination. Nevertheless, whereas humans and other Old World anthropoid primates have three types of retinal cones, ancestral anthropoids probably possessed two types of cones, similar to most modern strepsirrhines and New World monkeys. A third cone type, enabling full trichromatic vision, appears to have evolved independently in the ancestors of Old World anthropoids and in several New World monkey groups. The enhancements of color vision in the anthropoids are plausibly adaptations for distinguishing ripe, edible fruits. The shift to diurnal activity was also marked by larger social groupings, which may offer enhanced protection from predation. These changes were accompanied by increased brain size, including increases in the temporal lobe visual region, and in regions of the parietal and frontal cortex mediating motor control and social interactions.
The ancestors of present-day tarsiers evidently abandoned the diurnal niche to become nocturnal visual predators again. Tarsiers retain a fovea, but they have a rod-dominated retina and their enormous eyes are sensitive at low light levels without the aid of a reflecting tapetum. The primary visual cortex became very large relative to the rest of the brain and is extremely well differentiated, with a multiplicity of layers and sublayers (Wong, Collins, & Kass, 2010). Tarsiers became such extremely specialized visual predators that they eat no plant food. Other haplorhines (anthropoids) remained diurnal and spread to many niches, including those outside the rainforest and niches based more on eating leaves as well as fruits. Some increased considerably in body size. Later anthropoids were able to process hard, husked fruits with their hands and teeth. Some early anthropoids managed to reach South America from Africa, apparently by rafting, to form the New World monkey radiation. All modern anthropoids are diurnal with the exception of owl monkeys, a New World monkey group that has (like tarsiers) become secondarily nocturnal.
In Africa, early anthropoids diverged some 25 to 30 million years ago into lines leading to modern Old World monkeys and to apes. At first, apes were the most successful radiation, coming to occupy a range of rainforest and open woodland environments, while monkeys were quite rare. This condition changed radically some 10 million years ago when monkeys became abundant and apes rare. This change may have resulted from the advent of a more seasonably variable and drier climate. Ancestral Old World monkeys acquired specialized teeth suited to a leaf and fruit diet in drier climates. Also, the more rapid reproduction of monkeys may have made them more resistant to extinction than apes.
Some 6 to 8 million years ago, a line of apes diverged into two branches: one that gave rise to modern common chimpanzees and bonobos (pygmy chimpanzees) and a second branch that led to the group of bipedal apes that includes modern humans, the “hominins” (formerly known as “hominids,” a term that now is used to refer to the great ape-human group) (Fig. 42.5).
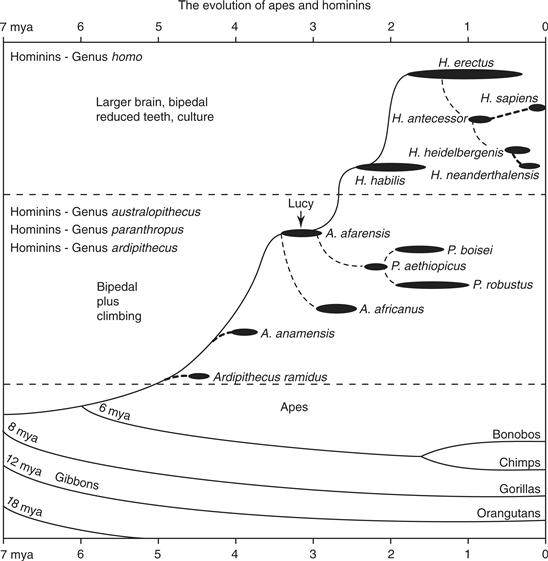
Figure 42.5 The evolution of apes and humans. Hominins include those extinct species that are judged to have been bipedal. Ardipithecus ramidus is generally thought to be bipedal while Australopithecus and Paranthropus clearly appear to have been bipedal. The age of the famous fossil, Lucy, is indicated. Relationships are somewhat uncertain, and more branches on the tree exist (Wood & Lonergan, 2008).
Although the hominins today are represented by only one species, Homo sapiens, they were a remarkably successful and diverse group (de Sousa & Wood, 2007; Wood & Lonergan, 2008). The oldest known hominin radiation, australopiths, dates back at least 5 million years. These early hominins were bipedal, but skeletal traits suggest they retained considerable ability for climbing trees. Body size was rather small, and males were much bigger than females. Their brains were only slightly larger than those of apes of similar body size. Hominin traits may have emerged as adaptations to a drier environment with grassland and savanna. Early australopithecines soon gave rise to a number of species differing in body size and limb proportions, as well as in characteristics of the jaws and teeth and brain size. Our own genus emerged some 2 million years ago with Homo habilis (or “handy man,” due to its use of stone tools). Homo habilis had a slightly increased cranial capacity compared to australopithecines, reduced face and teeth, and pelvic modifications for improved bipedal locomotion and the birth of neonates with larger heads. About 1.7 million years ago, H. habilis appears to have been replaced by H. erectus, a larger hominin with a further reduction in face and teeth and a larger brain. Shortly thereafter, H. erectus spread out of Africa to central Asia. Homo sapiens emerged from an African population of H. erectus some 250,000 to 300,000 years ago. Other members of the genus Homo coexisted with H. sapiens until recently. The Neanderthals, who adapted to a colder Europe and southwest Asia over 130,000 years ago, disappeared and were replaced by modern H. sapiens some 35,000 years ago. Neanderthals were shorter and more heavily built than modern humans, but had a comparable or slightly larger cranial capacity. As another known example, the fossilized bones of a probable descendant of H. erectus, H. florensensis, have been dated to 18,000 years old (Morwood et al., 2004). Homo florensensis were small (one meter tall and 20 Kg.) with brains (380 grams) smaller than those of the great apes. Over the last 15,000 years, some populations of modern humans have become smaller and have reduced their brain size, possibly as a result of a poorer diet as agriculture replaced hunting and gathering.
Chimpanzees and bonobos (also misleadingly known as “pygmy chimpanzees”) appear to be our closest surviving relatives. Humans and chimpanzees are 98 to 99% similar in the coding sequences of genes, although the overall difference in DNA is closer to 96%, the difference reflecting the independent duplication, insertion, and deletion of DNA segments in the two lineages, and changes in noncoding regions that control gene expression. At present, we do not understand what changes in DNA account for the differences in brain size, anatomical organization, and function between humans and other apes, but this is an active area of research.
Presently, primates constitute a highly diversified taxonomic group (clade) that includes 14 families and over 350 species. Describing their brains is a challenging task, as the brains of only a few primate species have been extensively studied. It is clear, however, that primate brains vary enormously in size, and much of that variation reflects the 5000-fold range of body sizes found in primates—from the 40-gram mouse lemur to the 200-kilogram male gorilla (Fig. 42.6). Nevertheless, there are important differences in brain size that cannot be attributed to differences in body size. These variations in brain size reflect changes in the number of cortical areas and the size of individual areas. In addition, however, the internal architecture and intrinsic connectivity of areas were modified by evolution, as were the ways in which cortical areas are interconnected, reflecting the ways in which primates have diversified behaviorally. Despite these variations, primate brains also share a large number of features, some of which have been retained from nonprimate ancestors, and some of which emerged in early primates and have been widely retained.
Box 42.2 Why Brain Size is Important
Larger brains are generally thought to be computationally better because they usually have more neurons. However, growing bigger brains with more neurons creates a need for modifications in brain organization, and some solutions are likely to be common across taxa, allowing predictions about brain organization with brain size (Kaas, 2000). Enlarging a brain by having more neurons creates two related problems. If brain functions depend on each neuron maintaining the same proportion of connections with other neurons, then more neurons means more connections, which would be longer and take up more space. In addition, the conduction times of longer axons and dendrites would be longer, unless the thickness of the axons and dendrites were increased with the added length (Bekkers & Stevens, 1970). Thus, larger brains would have larger neurons with longer, thicker axons and dendrites. This suggests that brains would reach a maximum size, perhaps not much larger than that of present-day humans, because brain connections and glia would take up so much space with the addition of more neurons that such additions would be impractical. However, a design solution for such connection problems is for brains to become increasingly modular with larger size, so that short distance connections predominate, and only a few neurons have the long connections that are needed to coordinate the modular parts. Rodents do seem to increase the average neuron size with brain size, so that neuron densities greatly decrease as proportionately more space is devoted to glia and connections, but primates maintain neuron densities with increasing brain size, implying that average neuron size does not increase (Herculano-Houzel, Collins, Wong, & Kass, 2007). No other mammal, including elephants and whales with much larger brains, has the same number of neurons as the 1.5-kg human brain. A rodent with 86 billion neurons would need a 35-kg brain, well beyond the largest known brain mass of 9 kg for the blue whale (Herculano-Houzel, 2009). Whales and elephants may have evolved more modular brains as they acquired large brains, but they do not seem to have done it to the extent that seems to have occurred in the evolution of the large human brain. Over the course of the evolution of the large human brain, the neocortex of primates, which makes up the bulk of the human brain, acquired more cortical areas and more modular subdivisions of areas as brain sizes increased. As an example, the hemispheric specializations of the human brain presumably reduce the need for long connections between the two hemispheres.
A related issue is that large cortical areas are unlikely to function in the same manner as small cortical areas. Unless neurons compensate with larger dendrites and longer intrinsic connections as areas get larger, the computational window or scope of neurons will decrease (Fig. 42.10). For example, as a visual area gets bigger, individual neurons would evaluate information from less and less of the total visual field. This implies that as areas get bigger, their neurons become less capable of global center-surround comparisons and more devoted to local center-surround comparisons. Thus, some of the integrative functions of large areas must be displaced to smaller areas. The large V1 of the human brain preserves the detail of visual scenes, but perception depends on other, smaller visual areas. It is also apparent that changes in the sizes of dendritic arbors and the lengths of intrinsic axons in smaller areas would have more impact on the sizes of computational windows of neurons. Comparable changes in dendrites and axons would enlarge or reduce receptive field sizes more in a small than a large visual area. Because their functions are more modifiable by smaller structural modifications, smaller areas may be specialized more easily for different functions. Neurons in large areas typically do not have larger dendritic arbors and longer intrinsic connections, and indeed, they may have smaller dendritic arbors. Thus, V1 of primates has overall the smallest and most densely packed neurons of all cortical areas, and this is especially the case in large-brained anthropoid primates (Collins, Airey, Young, Leitch, & Kaas, 2010). In addition, although primary sensory areas are often larger in larger brains, they are not enlarged in proportion to the rest of cortex. Thus, in surface area primary visual cortex is less than three times larger in human brains than in the brains of macaque monkeys, whereas the neocortex as a whole is over 10 times larger. Neocortex in chimpanzee brains is one-fourth the size of the human neocortex, while primary visual cortex (V1) is about the same size. The general lack of proportional growth of cortical areas with brain size reduces the impact of the changing functions of areas with size, and reflects the addition of other smaller cortical areas.
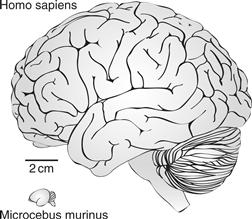
Figure 42.6 Lateral views of the brains of a human and a small prosimian primate, the mouse lemur, to illustrate the great range of sizes for present-day primates.
Brains of Early Primates
Early primates resembled present-day lemurs and galagos in body form and brain shape, but the brains were significantly smaller in proportion to the body. Except for a lateral fissure and a calcarine fissure, features of the brains of most primates, there were only a few shallow fissures. We know most about the organization of neocortex and other parts of the brain of early primates from studies of present-day strepsirrhine primates, especially galagos, as they have been most extensively studied. The brains are smaller in proportion to body size than the brains of anthropoid primates, and they contain a number of distinctive features that are also found in the brains of other primates. While our focus is on cortical organization, we will also briefly describe subcortical features that were likely present in early primates, especially those for sensory systems.
Vision became a more important sense in early primates (Kaas, 2007b) as they came to rely less on olfaction and more on detecting prey and other food visually, and using eye-to-hand coordination to navigate in the fine branches of bushes and trees and bring food objects to the mouth. Compared to the nearest relatives of primates (tree shrews, flying lemurs, rabbits, and rodents), early primates had more forward-facing eyes, creating a larger binocular field of 130 degrees or more and a shift of the retinal region of high receptor and ganglion cell density toward the center of the retina. These modifications permitted a fine-grain level of stereopsis in central vision and the detection of otherwise cryptic invertebrate prey. The retina had the three major classes of ganglion cells that are found in all extant primates; the small koniocellular (K), medium parvocellular (P), and large magnocellular (M) ganglion cell classes, which are named after their layers of termination in the lateral geniculate nucleus of the visual thalamus. Detailed vision depends on the P cells, which constitute the majority of retinal ganglion cells in all primates. K cells constitute only about ten percent of the ganglion cells in diurnal primates, but they are more common in nocturnal primates, and would have been so in early primates since they were nocturnal. M cells respond to visual change such as that commonly produced by object movement. M cells represent roughly ten percent of the ganglion cells. The ganglion cells project to a number of small brainstem targets, as well as to the dorsal lateral geniculate nucleus (LGN) and the superior colliculus (SC).
Early primates and all subsequent primates differed from their nonprimate ancestors by representing only the contralateral visual hemifield via the nasal retina of the contralateral eye and the temporal retina of the ipsilateral eye in the superior colliculus. Nonprimate mammals preserve the ancestral mammalian condition, in which the complete retina of the contralateral eye projects to SC. This modification in primates likely promoted the coordination of eye movements and binocular vision.
The other major target of the retina, the LGN, also became specialized with the first primates. A common pattern of four main layers emerged with two ventral magnocellular layers and two dorsal parvocellular layers, with the outer layers for the contralateral eye and the inner layers for the ipsilateral eye. As early primates were nocturnal, they also likely had two thin koniocellular layers between the P layers, one for each eye, as in all extant strepsirrhines. These layers did not persist in anthropoid primates, in which the K cells remained but were scattered between the other layers. In many anthropoid primates, including humans, the P-cell layers became massive, and subdivided into four or more sublayers that allowed further emphasis on detailed object vision. The visual pulvinar was also organized in a distinctive primate specific pattern, with an inferior pulvinar of at least three divisions and a lateral pulvinar of possibly two divisions.
In early primates, the fossil record indicates that visual cortex occupied a large portion of neocortex as occipital cortex extended caudally to cover the midbrain and much of the cerebellum, and temporal cortex extended forward and down as in present-day galagos (Fig. 42.7). This visual cortex was divided into a number of visual areas, some of which appear to be unique to primates. Other visual areas retained from nonprimate ancestors became modified to subserve the increased role for vision in early primates.
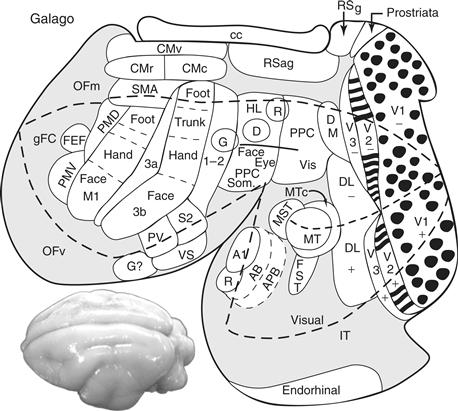
Figure 42.7 A surface view of the flattened neocortex of a prosimian primate, Galago garnetti, showing some of the proposed visual, somatosensory, auditory, and motor areas. Visual areas include the primary (V1) and secondary (V2) areas, common to most mammals, but with the modular subdivisions (blobs in V1; bands in V2) characteristic of primates. As in other primates, galagos have a third visual area (V3), a dorsomedial area (DM), a middle temporal area (MT), a dorsolateral area (DL), a fundal-sucal-temporal area (FST), a middle superior temporal area (MST), and an inferior temporal visual region (IT) with subdivisions. Posterior parietal cortex (PP) contains a caudal division with visual inputs and a rostral division with sensorimotor functions. The auditory cortex includes a primary field (A1), a rostral area (R), and an auditory belt (AB), which includes several areas, and parabelt auditory cortex (APB). The somatosensory cortex includes a primary area (S1 or 3b), a parietal ventral area (PV), a secondary area (S2), a parietal ventral area (PV), a somatosensory rostral belt (RS or 3a), and a somatosensory caudal belt (CS or possibly area 1 or areas 1 plus 2). A gustatory area (G?) may have been present. Motor areas include a primary area (M1), ventral (PMV) and dorsal (PMD) premotor areas, a supplementary motor area (SMA), a frontal eye field (FEF), and other motor areas on the medial wall of the cerebral hemisphere. The thick dashed line encloses cortex that can be seen on a dorsolateral view of the intact brain (lower left). Granular frontal cortex (gFC); orbital frontal medial cortex (OFm); orbital frontal ventral cortex (OFv); ventral, rostral, and caudal cingulate motor areas (CM); retrosplenial granular and agranular areas (RSg and RSug); corpus callosum (CC). Modified from Kaas (2007b).
The arrangement of some of the visual areas that were likely present in the visual cortex of early primates are shown on a surface view of the neocortex of one hemisphere of a galago brain, after the neocortex has been separated from the rest of the brain and flattened so that hidden surfaces are visible (Fig. 42.7). As in extant primates, early primates had three early stages of cortical processing: V1, V2, and V3. V1 was large and densely packed with small layer 4 cells, more so than any other cortical area (Collins et al., 2010), as a way to promote detailed vision. In primates, but not in the close relatives of primates, V1 has a distribution of cytochrome oxidase “blobs” that may represent a specialized output of the K-geniculate layers (Casagrande & Kaas, 1994). All primates also have an orderly arrangement of direction-of-motion and stimulus-orientation neurons in V1 that look like pinwheels from the surface. This feature of V1 likely predated the evolution of primates, as the close relative of primates, tree shrews, also have orientation-specific pinwheels in V1, but V1 of more distantly related rodents and lagomorphs does not. The segregation of eye of origin information into ocular dominance bands or patches is also a specialization in V1 of primates, being absent in tree shrews, rodents, and rabbits. Ocular dominance columns evolved independently in carnivores, as did blobs.
V2 is another area that is common to most mammals, while being specialized in primates by having three repeating classes of band-like modules that cross the width of V2, the so-called thin, pale, and thick stripes, each receiving a different type of input from V1 and projecting differently to higher order visual areas. These modules exist in strepsirrhine as well as in anthropoid primates, but they are much more histologically distinct in anthropoids. These band-like modules are not found in the relatives of primates, suggesting that they evolved in early primates and were further differentiated in anthropoids.
V3 also exists in all studied primates, although its existence as a field bordering V2, with representations of both the upper and lower visual quadrants, has been questioned. There is no compelling evidence yet for a V3 in the close relatives of primates, despite the clear evidence for a V3 type of area in carnivores, so V3 may be a primate specialization. However, V3 has only recently been well characterized in primates, and the close relatives of primates should be further investigated.
The middle temporal visual area, MT, is a visual area that is recognized widely as being present in all primates. MT receives most of its activation inputs from the M ganglion cell population via direct and indirect projections from V1, and is highly involved in analyzing visual motion. MT is part of a constellation of functionally related areas, MST, FST and MTc (V4t), that all appear to be unique to primates, although homologues of MT in other mammals have been suggested. The dorsolateral visual area (DL or V4) is another area that may be a primate specialization, as is the dorsomedial visual area (DM or V3A). A number of other visual areas have been proposed, especially for macaque monkeys and humans. But for the most part, these areas have not been well defined across a range of primate species. Nevertheless, primates share as many as 10 to 15 visual areas. All primates appear to have their visual systems organized into dorsal and ventral streams of visual processing for guiding actions and object vision, respectively. Dorsal-stream motion-sensitive visual areas such as MT project to posterior parietal cortex and object related visual areas such as DL/V4 project to visual areas of the inferior temporal lobe. Both parietal and temporal areas relay to frontal cortex (Preuss & Goldman-Rakic, 1991).
The auditory system has been less extensively studied than the visual system in primates, and subcortical processing stations such as the inferior colliculus and medial geniculate complex are assumed to be organized in a manner similar to those of other mammals. However, mammals vary considerably in the arrangement of auditory cortical areas (Kaas, 2011b). Primates are generally thought to have three early stages of auditory processing, a primary core, a surrounding belt, and an adjoining parabelt (Kaas & Hackett, 2000). The primary core in all primates appears to include at least two areas: A1 and a rostral area, R. Both represent tone frequencies from high-to-low, and have the histological, connectional, and functional properties of primary sensory cortex. Little is known about how belt and parabelt regions are organized across primate taxa, or in any strepsirrhine primates, so little can be inferred about early primates. However, given the presence of at least two primary areas in rodents, as well as adjoining secondary areas, early primates likely retained two or more primary areas and some secondary areas from earlier nonprimate ancestors.
The somatosensory system of early primates was likely similar to those of other mammals at early stages of processing. The system for object recognition included the dorsal-column-medial lemniscus pathways to the ventroposterior nucleus of the somatosensory thalamus. This pathway was more devoted to the tactile receptors of the glabrous skin of the forepaw (hand) than in most mammals, and this skin had larger representations at thalamic and cortical levels. Nevertheless, the thalamic and cortical representations of the face, teeth, and tongue were quite large, with representations of both ipsilateral and contralateral teeth and tongue. The area generally recognized as area 3b (S1 proper) in anthropoid primates constitutes the homolog of nonprimate S1, although it is common in descriptions of somatosensory cortex in humans to include areas 3a, 3b, 1, and 2 in “S1.” In strepsirrhines and some New World monkeys, only one representation of the contralateral body has been found caudal to area 3b, so it is not clear if this region is homologous to area 1 or to both areas 1 and 2. Here we propose that early primates had a single area, corresponding to areas 1 plus 2 of anthropoid primates. Area 3a is a proprioceptive area with inputs from the ventroposterior superior (VPS) nucleus of the somatosensory thalamus. Area 3a, or dysgranular somatosensory cortex was likely retained from early mammals, as were areas S2, PV, and gustatory cortex (G). The ventral somatosensory area (VS), with two divisions, appears to exist in most primates, but has not been identified in other mammals.
Primary motor cortex (M1) and likely one or more premotor areas are found in all primates and were retained from nonprimate ancestors, as M1 and a premotor area are found in rodents and tree shrews (Remple, Reed, Stepniewska, Lyon, & Kaas, 2007). All primates also have dorsal and ventral premotor areas (PMD and PMV), a supplementary motor area (SMA), and two or more cingulate motor areas. The arrangement of these motor fields is remarkably consistent across primate taxa, although premotor areas clearly have functionally distinct subdivisions in some anthropoid primates. Frontal cortex has retained divisions of orbital frontal cortex, while the granular prefrontal cortex is unique to primates (Preuss, 1995). Granular prefrontal cortex is small in strepsirrhines but includes several areas common to primates, including the frontal eye field (FEF). The prefrontal cortex underwent substantial enlargement and elaboration in anthropoid evolution, and areas like the principalis cortex (area 46) of macaques, which mediate higher cortical functions, are most likely anthropoid or catarrhine specializations.
Another region that appears to have changed markedly with the origin of primates is posterior parietal cortex (PPC). In rodents and tree shrews there is little cortex that could be defined as posterior parietal cortex, and visual and somatosensory information reaches motor cortex more directly. However, all primates have a large region of posterior parietal cortex, and part of this cortex has an orderly arrangement of functionally distinct zones that are involved in reaching, defensive, grasping (zones G, D, and R in Fig. 42.7) and other movement sequences (Kaas, Gharbawie, & Stepniewska, 2011). In strepsirrhine galagos, the caudal half of PPC receives most of the visual input, while projecting to the movement-related zones in the rostral half of PPC. Rostral PPC has dense somatosensory inputs, and projects to functionally matched zones in motor and premotor cortex. The organization of PPC is more complex in anthropoid primates, especially in humans where PPC is significantly involved during tool use. PPC also has strong connections with granular prefrontal cortex, and with the higher-order multisensory cortex of the temporal lobe. In primates, these three cortical regions are connected to a common thalamic nucleus, the dorsal pulvinar (also known as the medial pulvinar), and like these cortical regions, the dorsal pulvinar has no obvious homolog in nonprimate mammals (Preuss, 2007).
Brains of Early Anthropoids
The anatomy of early anthropoids suggests that an emphasis on high-acuity, diurnal vision and a reduced emphasis on smell were important in their evolution. The endocasts of the skulls of early anthropoids indicate that the visual cortex of the temporal lobe was more expansive than in strepsirrhines, and overall the brains were considerably larger relative to body size. After the divergence of New World and Old World anthropoids, brain size increased independently in both groups. New World monkeys cover a particularly large range of body and brain size. Since the degree of folding depends on the absolute size of the brain, the smaller-brained New World monkeys like marmosets, owl monkeys, and squirrel monkeys have relatively smooth brains, while the larger species, such as capuchins, spider monkeys, and howler monkeys, have well-fissured brains that resemble (at least superficially) the convoluted brains of Old World monkeys
Both Old World and New World monkeys have all of the areas of the neocortex described for strepsirrhines, as well as some additional areas. Most notably, the somatosensory cortex has been altered so that areas 3a, 3b, 1, and 2 are well differentiated from each other, with each area corresponding to a separate representation of receptors of the contralateral body surface (Kaas, 2007b). Within S1 (area 3b), the proportional representation of body parts varies somewhat across species, so that in some monkeys as much as half of the area is devoted to the face and oral cavity. The hand is also prominently represented, especially in monkeys such as macaques. Some New World monkeys, such as spider monkeys, have a large representation of the hairless (glabrous) ventral skin of their highly tactile, prehensile tail. Marmosets appear to lack a separate area 2, a field that is responsive to tactile stimuli and muscle movements in other monkeys. This may be a consequence of brain size reduction in marmosets, which have evolved smaller brains and bodies from larger ancestors, and transformed their nails back into claws. In all anthropoids, S2 and PV appear to have lost activating inputs from the ventroposterior nucleus of the somatosensory thalamus, and they depend on inputs from areas 3a, 3b, 1, and 2 (Fig. 42.8). S2 and PV receive modulatory inputs from the ventroposterior inferior nucleus. Thus, processing in the somatosensory system became more serial than parallel with the advent of monkeys. Other differences likely exist between anthropoids and strepsirrhines in the somatosensory portions of the lateral sulcus, and more research is needed.
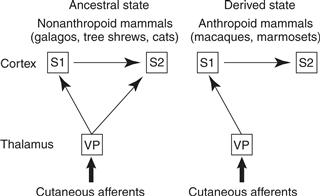
Figure 42.8 Somatosensory processing in prosimian primates and anthropoid primates. The processing in anthropoids is serial, rather than parallel and serial. Because the prosimian type is also found in a number of nonprimate mammals, we infer that this is the ancestral state and the anthropoid type is the derived or newly evolved state. The ventroposterior nucleus (VP) of the somatosensory thalamus and the first (S1) and second (S2) somatosensory areas of the cortex are shown.
Currently, there is much interest in how the visual cortex of monkeys is subdivided into areas and how these visual areas function in behavior (Orban, Van Essen, & Vanduffel, 2004). Elaborate proposals have been presented, but considerable uncertainty remains. In Old World monkeys, over 30 visual areas have been proposed, and it seems likely that anthropoids in general have more visual areas than strepsirrhines, although the full extent of this difference is not yet clear. The auditory cortex of strepsirrhines has been poorly studied, and it is unclear whether they share the same core-belt-parabelt organization that has been identified in anthropoids. The proposed subdivisions of the motor cortex in strepsirrhines and anthropoids are quite similar, although some of the premotor areas of strepsirrhines have been subdivided into two or three areas in Old World monkeys. Finally, as noted above, the higher-order temporal, parietal, and prefrontal cortex was greatly expanded in anthropoid evolution, and comparative studies of (strepsirrhine) galagos and (anthropoid) macaques indicate that macaques possess a number of areas in prefrontal cortex that galagos lack. The same is likely true of the parietal and temporal cortex, but these regions have not been compared as comprehensively as prefrontal cortex.
Evolution of Hominin Brains
Understanding human brain evolution requires determining what changes took place in the human brain in the 6 to 8 million years since the human and chimpanzee-bonobo lineages diverged. For a long time, nearly all one could say about these changes is that human brains got larger (Box 42.2), reaching about three times the volume of those of chimpanzees and other great apes, although humans are only slightly larger than chimpanzees in adult body size (Fig. 42.9). From the fossil record, we also know that most of this increase took place in the last ~2 my, with the emergence of genus Homo, and that there were several species of this genus with brains as large as our own, although only Homo sapiens remains today. But the fossil record cannot tell us much about what went on inside the brain, which is what we really want to know if we are to understand the evolution of human-specific behavioral and cognitive abilities, such as language and the ability to represent and reason about abstract or invisible causes, such as mental states (Povinelli & Preuss, 1995; Premack, 2007).
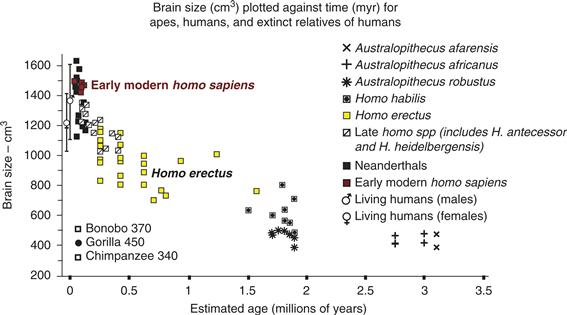
Figure 42.9 Evidence for the rapid growth of brains of hominins over the last two million years. The brain sizes of modern chimpanzees and gorillas have been added for comparison. Modified from McHenry (1994).
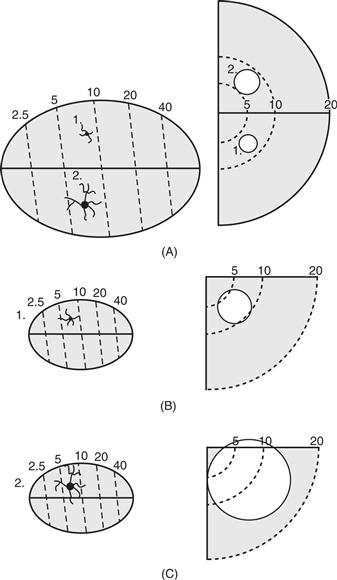
Figure 42.10 The effects of varying the horizontal spread of dendritic arbors of neurons in large (A) and small (B and C) visual areas. An increase in arbor size (1 to 2) in a large area (A) produces little change in receptive field size (circles 1 to 2 in the central 20° of the visual hemifield on the right), whereas such a change (B to C) in a small area changes the scope of the receptive field greatly. Thus, the functions of small areas are changed more dramatically by small morphological adjustments. Surface view schematics of retinotopically organized visual areas are on the left, whereas schematics of receptive fields in the visual hemifield and the lower visual quadrant are on the right. From Kaas (2000).
To understand changes in internal organization, we need to compare human and ape brains directly and identify the ways in which they differ. This has posed a great challenge, because neither humans nor apes can be studied with the invasive and terminal techniques so widely used with model animals in the neurosciences. With the development of noninvasive neuroimaging techniques, however, along with the proliferation of antibodies and other molecular tools for probing brain structure in postmortem material, and the rise of comparative genomics, the comparative approach to studying human brain evolution has entered a new and productive era.
Traditionally, it was postulated that the higher-order regions of the frontal, parietal, and temporal lobes (the classical “association” cortex) underwent great expansion in human evolution. This view was recently called into question by comparative structural neuroimaging studies that found that the relative proportions of the frontal, parietal, and temporal lobes are similar in great apes and humans, notwithstanding the much larger absolute size of the human brain. The fact remains, however, that the primary sensory cortical and thalamic structures are of roughly the same absolute size in humans and great apes, while other regions of cortex in humans are much larger in absolute terms. This is consistent with classical accounts of human brain evolution that emphasize the expansion of higher-order regions (Preuss, 2011).
One of the signature specializations of H. sapiens is of course language, and it has often been assumed that the evolution of language involved the evolution of specialized language areas in the brain, such as Wernicke’s area in the temporoparietal cortex and Broca’s area in the frontal lobe. Current evidence, however, strongly suggests that homologues of Wernicke’s and Broca’s areas are present in apes and monkeys, which in turn suggests that evolution “recruited” these areas and modified them to support language functions. A recent comparative neuroimaging study with diffusion tensor imaging (DTI) has demonstrated that the major pathway between the frontal and temporal language territories (the arcuate fasciculus) has different connectivity patterns in humans and chimpanzees, with humans showing much stronger connections with the middle temporal gyrus, which in humans is involved in representation of word meanings (Rilling et al., 2008). This is one of several pieces of evidence indicating that the middle temporal cortex expanded greatly in human evolution, displacing the temporal lobe visual areas posteriorly and inferiorly in humans, compared to their location in monkeys.
Since most humans are left-hemisphere dominant for language, it is natural to assume that human brains are more strongly lateralized than those of apes. Surprisingly, apes share some of the gross asymmetries of brain morphology found in humans, including a larger planum temporale (the region of superior temporal cortex often identified with Wernicke’s area) on the left than the right. Microscopic studies of postmortem tissue, however, indicate that Broca’s area, which is larger on the left in humans, is not asymmetrical in size in chimpanzees.
Humans are unusual among mammals in their facility in making and using tools. Functional imaging studies in humans reveal that these activities tap specific, higher-order regions of parietal and frontal cortex (Stout, Passingham, Frith, Apel, & Chaminade, 2011). Direct comparison of brain activity in humans and macaques during tool use indicates that humans have a small territory of left inferior parietal cortex that is selectively responsive when humans view tools that are used to grasp objects, whereas macaque neural activity does not differ between viewing grasping actions of hands and tools (Peeters et al., 2009). It is unclear whether the tool-specific area of humans is a novel area or a modified form of an area present in other primates. A similar issue is raised by the observation (Vanduffel et al., 2002) that the cortex of the intraparietal sulcus is much more responsive to certain types of object movement in humans than in macaques: are these new areas or modified areas? We cannot answer these questions without more detailed comparative studies, with studies of chimpanzees being especially important. In any case, however, it is likely that human cortex is tuned to dimensions or aspects of movement in ways that distinguish humans from at least most other anthropoid primates.
Microscopic studies have also identified specializations of cellular and molecular biology of humans. For example, humans and great apes possess a distinctive type of large, bipolar cell (spindle cells or Von Economo neurons) in anterior cingulate and insular cortex. While present in apes as well as humans, they are especially large and numerous in humans. Similar cells are not found in other anthropoid primates. Interestingly, a similar cell type has evolved independently in whales and elephants, suggesting that this morphology is part of an adaptation for rapid neural communication in large-brained animals (Allman et al., 2011; Watson & Allman, 2007).
The previous example illustrates that evolution can modify areas that are phylogenetically ancient, and not only areas that are evolutionarily new or functionally higher-order. Another example comes from primary visual cortex. Several lines of evidence suggest that apes and humans reduced or lost a projection from the lateral geniculate nucleus to cortical layer 4A, the upper part of cortical layer 4. (Comparative studies indicate that the upper parts of layer 4 of anthropoids, 4A and 4B, are actually parts of layer 3; see Casagrande and Kaas (1994). In addition, humans further modified layer 4A, adding a novel set of compartments that stain for molecules usually associated with the magnocellular processing stream, the part of the visual system involved in motion analysis (Preuss & Coleman, 2002; Preuss, Qi, & Kaas, 1999). These modifications could influence the functional properties of higher-order areas, including the intraparietal cortex, since V1 is the major source of visual information for the rest of the cortex.
Another new area of active research that provides information about human brain evolution comes from comparative genomics, the goal being to identify evolutionary changes in gene sequences and gene expression relevant to human brain evolution. Although it is commonly assumed that humans and chimpanzees are nearly identical genetically, the most recent genomic comparisons have revealed a plethora of differences, including hundreds of adaptive changes in protein coding sequences; myriad deletions, duplications, and insertions of blocks of DNA; changes in transcription factor binding sites; and differences in the amounts of specific genes and proteins expressed in the brain (Varki & Nelson, 2007). At present, we do not understand what changes in DNA account for the differences in brain size, anatomical organization, and function between humans and other apes, but this is an active area of research. There is, however, intriguing evidence suggesting that the expression of genes involved in synaptic plasticity was modified in human evolution, and that human brains are adapted to sustain unusually high rates of energy metabolism (Cáceres et al., 2003; Fu et al., 2011; Preuss, 2011).
Conclusions
Based on comparative studies and the fossil record, we conclude that early mammals had small brains with little neocortex and few functional subdivisions (areas or fields) of cortex. Vision was emphasized in the early primates, and the visual cortex in the temporal and occipital lobes was enlarged. These primates also had several unique features of the visual system, including new visual areas such as MT, distinctive kinds of modules in V1 (blobs) and V2 (bands), separate magnocellular and parvocellular layers in the lateral geniculate nucleus, and a representation in the superior colliculus restricted to the contralateral visual hemifield. Several premotor areas were present, whereas the somatosensory system was relatively primitive. Later anthropoid primates had larger brains, more neocortex, and more areas of neocortex. The somatosensory cortex had expanded and included the four strip-like areas on the anterior parietal lobe, areas 3a, 3b, 1, and 2. We know little about possible specializations of the brains of apes. However, over the last 6 million years of evolution in the human lineage, brains increased three to four times in size, due mainly to the enlargement of the cortex. Although the relative proportions of the different cortical lobes remain similar in humans and apes, the available evidence suggests that the cortex did not expand uniformly in human evolution, as the higher-order regions of frontal, temporal, and parietal lobes are much larger in absolute terms in humans than in apes, while human primary sensory areas are about the same absolute size as those of apes. The expansion of nonprimary cortex was probably accompanied by a further increase in the number of cortical areas, modifications leading to functional and anatomical asymmetries in the two cerebral hemispheres, specializations for language and cognition, and larger expanses of prefrontal, parietal, and temporal cortex. The cortex of the larger brains had many more neurons with a greater proportion of tissue devoted to subcortical white matter connections relative to cell bodies (Herculano-Houzel, Mota, Wong, & Kaas, 2010), and presumably had a higher ratio of local connections to long-distance connections.
Further progress in understanding the evolution of the human brain can be achieved with current methods of investigation. We have the opportunity to learn much about the similarities and differences among the brains of various primates. Neuroscientists have generally concentrated on studies of brain features that are widely shared, but we need to know more about the differences in brain structure and function that make us distinctively human.
References
1. Aiello LC, Wheeler P. The brain and the digestive system in human and primate evolution. Current Anthropology. 1995;36:199–221.
2. Allman JM. Evolving brains New York, NY: H. W. Freeman; 1999.
3. Allman JM, Tetreault NA, Hakeem AY, et al. The von Economo neurons in the frontoinsular and anterior cingulate cortex. Annals of the New York Academy of Sciences. 2011;1225:59–71.
4. Azevedo FA, Carvalho LR, Grinberg LT, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. Journal of Comparative Neurology. 2009;513:532–541.
5. Bekkers JM, Stevens CF. Two different ways evolution makes neuron larger. Progress in Brain Research. 1970;83:37–45.
6. Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrhinde Leipzig: Barth; 1909; [Reprinted as Brodmann’s Localisation in the cerebral cortex, (L. J. Garey, Trans. and Ed.). London: Smith-Gordon, 1994.].
7. Cáceres M, Lachuer J, Zapala MA, et al. Elevated gene expression levels distinguish human from non-human primate brains. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1330–1335.
8. Casagrande VA, Kaas JH. The afferent, intrinsic, and efferent connections of primary visual cortex in primates. In: Peters A, Rockland K, eds. New York, NY: Plenum Press; 1994:201–259. Cerebral cortex. Vol. 10.
9. Catania KC, Lyon DC, Mock OB, Kaas JH. Cortical organization in shrews: Evidence from five species. The Journal of Comparative Neurology. 1999;410:55–72.
10. Changizi MA, Shimojo S. Parcellation and area-area connectivity as a function of neocortical size. Brain, Behavior and Evolution. 2004;66:58–98.
11. Collins CE, Airey DC, Young NA, Leitch DB, Kaas JH. Neuron densities vary across and within cortical areas in primates. Proceedings of the National Academy of Sciences. 2010;107:15927–15932.
12. de Sousa AA, Wood B. The hominin fossil record and the emergence of the modern human central nervous system. In: Kaas JH, Preuss TM, eds. London: Elsevier; 2007;292–336. Evolution of nervous systems. Vol. 3.
13. de Sousa AA, Sherwood CC, Mohlberg H, et al. Hominoid visual brain structure volumes and the position of the lunate sulcus. Journal of Human Evoluation. 2010;58:281–292.
14. Falk D. Brain evolution in Homo: The “radiator” theory. Behavioral and Brain Sciences. 1990;13:339–381.
15. Finlay BL, Brodsky P. Cortical evolution as the expression of a program for disproportionate growth and the proliferation of areas. In: Kaas JH, Krubitzer LA, eds. London: Elsevier; 2007;73–96. Evolution of nervous systems. Vol. 3.
16. Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584.
17. Fu X, Giavalisco P, Liu X, et al. Rapid metabolic evolution in human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6181–6186.
18. Herculano-Houzel S. The human brain in numbers: A linearly scaled-up primate brain. Frontiers in Human Neuroscience. 2009;3:31 Epub 2009 November 9.
19. Herculano-Houzel S, Collins C, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3562–3567.
20. Herculano-Houzel S, Mota B, Wong P, Kaas JH. Connectivity driven white matter scaling and folding in primate cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19008–19013.
21. Jerison HJ. What fossils tell us about the evolution of the neocortex. In: Kaas JH, Krubitzer LA, eds. London: Elsevier; 2007;1–12. Evolution of nervous systems. Vol. 3.
22. Kaas JH. Why is brain size so important: Design problems and solutions as neocortex gets bigger or smaller. Brain and Mind. 2000;1:7–23.
23. Kaas JH. Reconstructing the organization of neocortex of the first mammals and subsequent modifications. In: Kaas JH, Krubitzer LA, eds. London: Elsevier; 2007a;27–48. Evolution of nervous systems. Vol. 3.
24. Kaas JH. The evolution of sensory and motor systems in primates. In: Kaas JH, Preuss TM, eds. London: Elsevier; 2007b;35–57. Evolution of nervous systems. Vol. 4.
25. Kaas JH. Reconstructing the areal organization of the neocortex of the first mammals. Brain, Behavior and Evolution. 2011a;78:7–21.
26. Kaas JH. The evolution of auditory cortex: The core areas. In: Winer JA, Schreiner CE, eds. The auditory cortex. New York: Springer-Verlag; 2011b;407–427.
27. Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11793–11799.
28. Kaas JH, Gharbawie OA, Stepniewska I. The organization and evolution of dorsal stream multisensory motor pathways in primates. Frontiers in Neuroanatomy. 2011;5:34 Epub 2011 June 13.
29. Martin RD. Primate origins: Implications of a cretaceous ancestry. Folia Primatologica (Basel). 2007;78(5–6):277–296.
30. McHenry HM. Tempo and mode in human evolution. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:6780–6786.
31. Morwood MJ, Soejono RP, Roberts RG, et al. Archaeology and age of a new hominin from Flores in eastern Indonesia. Nature. 2004;431:1087–1091.
32. Murphy WJ, Pevzner PA, O’Brien JO. Mammalian phylogenomics comes of age. Trends in Genetics. 2004;20:631–639.
33. Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends in Cognitive Sciences. 2004;8:315–324.
34. Peeters R, Simone L, Nelissen K, et al. The representation of tool use in humans and monkeys: common and uniquely human features. Journal of Neurosciences. 2009;29:11523–11539.
35. Povinelli DJ, Preuss TM. Theory of mind: evolutionary history of a cognitive specialization. Trends in Neurosciences. 1995;18:418–424.
36. Premack D. Human and animal cognition: continuity and discontinuity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13861–13867.
37. Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. The Journal of Cognitive Neuroscience. 1995;7:1–24.
38. Preuss TM. Evolutionary specializations of primate brain systems. In: Ravosa MJ, Dagasto M, eds. Primate origins: adaptations and evolution. New York, NY: Springer; 2007;625–675.
39. Preuss TM. The human brain: rewired and running hot. Annals of the New York Academy of Sciences. 2011;1225(Suppl 1):E182–E191.
40. Preuss TM, Coleman GQ. Human specific organization of primary visual cortex: Alternating compartments of dense Cat-301 and calbindin immunoreactivity in layer 4A. Cerebral Cortex. 2002;12:671–691.
41. Preuss TM, Goldman-Rakic PS. Architectonics of the parietal and temporal association cortex in the strepsirhine primate Galago compared to the anthropoid primate Macaca. Journal of Comparative Neurology. 1991;310:475–506.
42. Preuss TM, Qi H, Kaas JH. Distinctive compartmental organization of human primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:11601–11606.
43. Puelles L. Pallio-pallial tangential migrations and growth signaling: new scenario for cortical evolution?. Brain, Behavior and Evolution. 2011;78:108–127.
44. Radinsky L. Cerebral Clues. Natural History. 1976;85:54–59.
45. Remple MS, Reed JL, Stepniewska I, Lyon DC, Kaas JH. The organization of frontoparietal cortex in the tree shrew (Tupaia belangeri): II Connectional evidence for a frontal-posterior parietal network. Journal of Comparative Neurology. 2007;501:121–149.
46. Rilling JK, Glasser MF, Preuss TM, et al. The evolution of the arcuate fasciculus revealed with comparative DTI. Nature Neuroscience. 2008;11:426–428.
47. Ross C. Adaptive explanation for the origins of the Anthropoidea (Primates). American Journal of Primatology. 1996;40:205–230.
48. Shepherd GM. The microcircuit concept applied to cortical evolution: from three-layer to six-layer cortex. Frontiers in Neuroanatomy. 2011;5:1–15.
49. Stout D, Passingham R, Frith C, Apel J, Chaminade T. Technology, expertise and social cognition in human evolution. European Jouranl of Neuroscience. 2011;33:1328–1338.
50. Vanduffel W, Fize D, Peuskens H, et al. Extracting 3D from motion: Differences in human and monkey intraparietal cortex. Science. 2002;298:413–415.
51. Van Essen DC. Cerebral cortical folding patterns in primates: Why they vary and what they signify. In: Kaas JH, Preuss TM, eds. London: Elsevier; 2007;267–289. Evolution of nervous systems. Vol. 4.
52. Varki A, Nelson DL. Genomic comparisons of humans and chimpanzees. Annual Review of Anthropology. 2007;36:191–209.
53. Watson KK, Allman JM. Role of spindle cells in the social cognition of apes and humans. In: Kaas JH, Preuss TM, eds. London: Elsevier; 2007;479–484. Evolution of nervous systems. Vol. 4.
54. Wong P, Kaas JH. An architectonic study of the neocortex of the short-tailed opossum (Monodelphis domestica). Brain, Behavior and Evolution. 2009;73:206–228.
55. Wong P, Collins CE, Kaas JH. Overview of sensory systems of Tarsius. International Journal of Primatology. 2010;31:1002–1031.
56. Wood B, Lonergan N. The hominin fossil record: taxa, grades and clades. Journal of Anatomy. 2008;212:354–376.
Suggested Readings
1. Butler AB, Hodos W. Comparative vertebrate neuroanatomy New York, NY: Wiley-Liss; 1996.
2. Deacon TW. The symbolic species: The co-evolution of language and the brain New York, NY: Norton; 1997.
3. Falk D, Gibson KR. Evolutionary anatomy of the primate cerebral cortex Cambridge: Cambridge University Press; 2001.
4. Fleagle JG. Primate adaptation and evolution 2nd Ed. San Diego: Academic Press; 1999.
5. Gazzaniga MS. Human: The science behind what makes us unique 1st Ed. New York, NY: Ecco; 2008.
6. Kaas JH. In: Kaas JH, ed. London: Elsevier; 2007; Evolution of nervous systems. 4 Vols.
7. Passingham RE. What is special about the human brain? Oxford: Oxford University Press; 2008.
8. Preuss TM. The cognitive neuroscience of human uniqueness. In: Gazzaniga MS, ed. The cognitive neurosciences. 4th Ed., pp. 49–64 Cambridge, MA: MIT Press; 2009:49–64.
9. Preuss TM. Reinventing primate neuroscience for the twenty-first century. In: Platt ML, Ghazanfar AA, eds. Primate neuroethology. Oxford: Oxford University Press; 2010;422–453.
10. Sherwood CC, Subiaul F, Zawidzki TW. A natural history of the human mind: Tracing evolutionary changes in brain and cognition. Journal of Anatomy. 2008;212:426–454.
11. Striedter GF. Principles of brain evolution Sunderland, MA: Sinauer Associates; 2005.