9
TAKING CHARGE
The Policies and Research Funding We Need
Our lives begin to end the day we become silent about things that matter.
—Martin Luther King Jr.
ON FEBRUARY 22, 2006, AT THE WALTER E. WASHINGTON CONVENTION Center in Washington, DC, 3,200 people gathered for the World Parkinson Congress, the first major event to bring researchers and people with the disease together. The speakers included leaders from the National Institutes of Health (NIH), leading clinicians, and top scientists. And Tom Isaacs.
Eleven years before, Isaacs had been a twenty-six-year-old surveyor working for a London real estate company when he developed a tremor and was subsequently diagnosed with Parkinson’s. When he told his parents, he said, “I think I could be a good bartender. I have a really good shake.”
From the start, Isaacs was focused on a cure. His goal was to be among the first people to be able to say, “I used to have Parkinson’s.” At that time, he said, “the word cure was never used.… [Y]ou knew it was forbidden. Cure was seen as a false hope. And actually if you don’t have hope in Parkinson’s disease, you don’t have anything.”1
Isaacs left London on April 11, 2002, to start a different journey. Over the next year, he walked 4,500 miles around the coast of Great Britain—“anticlockwise,” as he put it. He climbed the highest mountains in England, Scotland, and Wales, wore through five pairs of boots, stayed at 238 different bed-and-breakfasts, walked with 1,032 different people, and ultimately raised $500,000 for Parkinson’s research (Figure 1). Days after finishing the journey, he ran the London Marathon.2
In 2005, Isaacs founded the Cure Parkinson’s Trust with three friends.3 They wanted “something focused on the research… and it needed to be a bit more edgy, a bit more feisty.”4 They did not just want to fund researchers and then wait for results. They wanted to drive progress.
Isaacs was relentless in his pursuit of a cure. He raised funds, met with countless scientists, visited Pope Francis to discuss the potential of stem cells (the Catholic Church opposes the use of stem cells derived from embryos but not other sources, such as adult stem cells), and volunteered to participate in clinical trials of experimental drugs.5 He was humorous, self-deprecating, and optimistic.
Isaacs believed that new treatments and a cure were only possible through the active participation of those affected by the disease. He said,
There is one thing that motivates me more than anything; it is the idea of finding a deliverable treatment that can reverse the course of this cruel illness. I believe that this is attainable. For me, one of the biggest challenges—and yet also the biggest opportunity—is galvanizing people who live with Parkinson’s from day to day to engage with their condition. If everyone with Parkinson’s were to communicate their experiences of living with Parkinson’s; if everyone participated in clinical trials; if everyone took the time to become more knowledgeable and was more committed to partnering [with] the scientific community in the search for new treatments, then there is no doubt in my mind that progress can be accelerated in Parkinson’s research.6
He established this partnership at his foundation, making sure that every research project it supported was evaluated by and relevant to people who had Parkinson’s. Though Isaacs died in 2017 at the age of forty-nine, the work at the trust continues as he intended—relentlessly, collaboratively, and joyfully.7
CHANGING POLICY TO PREVENT THE DISEASE
We can prevent many cases of Parkinson’s. Paraquat and trichloroethylene (TCE) are two of the biggest known culprits contributing to the rise in Parkinson’s, and the Environmental Protection Agency (EPA) has both the power and the responsibility to remove these threats. If it does, fewer of us will develop Parkinson’s. It is that simple.
In addition to the over 100,000 individuals who have signed a petition asking the EPA to ban paraquat, Marine veterans and their families are also demanding that the EPA ban TCE.8 They and their families have suffered terrible health consequences from the chemical and routinely go to Capitol Hill to pressure the EPA. Retired Master Sergeant Jerry Ensminger, who lost his daughter to leukemia at Camp Lejeune, said at a 2018 press conference, “What the hell are we waiting for? What is the EPA waiting for?”9 The EPA is failing to fulfill its core mission of “protecting human health and the environment [and of] providing clean and safe air, water, and land for all Americans.”10
In other parts of the government, there has been some movement. In 2016, as part of the 21st Century Cures Act, Congress authorized a National Neurological Conditions Surveillance System. In 2018, President Donald Trump signed a bill to fund the system with $5 million. The monies will initially be used to track and collect data on the number of people with Parkinson’s and multiple sclerosis.11 These efforts should help scientists identify geographic clusters of disease, evaluate potential risk factors, and analyze disease trends. While $5 million is a start, additional funds will be needed to support identification and assessments of risk going forward.
Looking for other risk factors is important, but it should not delay action on what we already know will lower all of our risk of developing Parkinson’s.
TURNING FRUSTRATION INTO ACTION
Dr. Andy Grove, the co-founder of Intel, understood that action had to be forceful. He dedicated much of the last years of his life to fighting Parkinson’s following his diagnosis in 2000.12
Grove was born to a Jewish family in Budapest, Hungary, in 1936. When he was eight, the Nazis occupied Hungary and deported half a million Jews to concentration camps. With a fake name and assistance from friends, Grove’s family escaped detection.13 Later, following the Hungarian Revolution of 1956, he and his family fled to Austria. They eventually made their way to the United States.
Six years later, Grove joined the pioneering chip manufacturer Fairchild Semiconductor. He later left to help form Intel, a company that he would eventually lead. Grove would transform the chip maker into an undisputed leader in Silicon Valley and become a respected thinker in business management circles.14
After Grove was diagnosed with Parkinson’s, he became frustrated by the lack of progress in terms of finding a cure. He said, “You can’t go close to this and not get angry. There are so many people working so hard and achieving so little.”15 True to the Silicon Valley way, Grove sought to disrupt the status quo. In a speech to several hundred of the world’s leading scientists, he said, “What is needed is a cultural revolution that values curiosity, follow-through and a problem-solving orientation.” He called on the scientists to put their data “in full view, scrutinizable by all.” By sharing data, researchers could minimize repeating experiments, avoid fruitless investigations, and build on the insights of other researchers.
He wrote to the NIH’s director about how to improve Parkinson’s disease research. He received no response, but that did not slow Grove.16 Unable to make changes at NIH, he made his own. With the University of California, he created new educational programs that married engineering principles with biological sciences.17 He pushed for objective measurement of Parkinson’s symptoms with new devices and wearable sensors.18 He insisted on data-sharing platforms. He gave millions to research.19 Grove was, according to the Washington Post, “an advocate and agitator for better and faster medical research.”20 His efforts have accelerated research cycles, broadened the sharing of research data, and spurred the use of technology in the Parkinson’s field.21
Many scientists benefitted from Grove’s mentorship. His style was blunt and direct.22 He guided hundreds and asked his protégés what their career goals were. If they were not clear enough, he would tell them to give it more thought. He told the cofounder of the Michael J. Fox Foundation, Deborah Brooks, “You have an obligation to behave exponentially different than everybody else.”23 Dr. Jeffrey Kordower, a leading Parkinson’s disease researcher at Rush University, said that Andy Grove “was incredibly direct and forced me to focus on problem solving and stay central to the core of what we were studying. He was an absolute hero. He changed the world.”24
Grove helped create Silicon Valley, developed new management principles, advanced cancer research (he survived prostate cancer), and provided educational scholarships for thousands around the world so that they can have the educational opportunities that he did.25 Upon his death, Grove dedicated $40 million of his estate to Parkinson’s research in partnership with The Michael J. Fox Foundation. Even without his resources, Grove’s dissatisfaction with the status quo drove change, an achievement that we can all strive for.
CLOSING THE FUNDING GAP IN PARKINSON’S RESEARCH
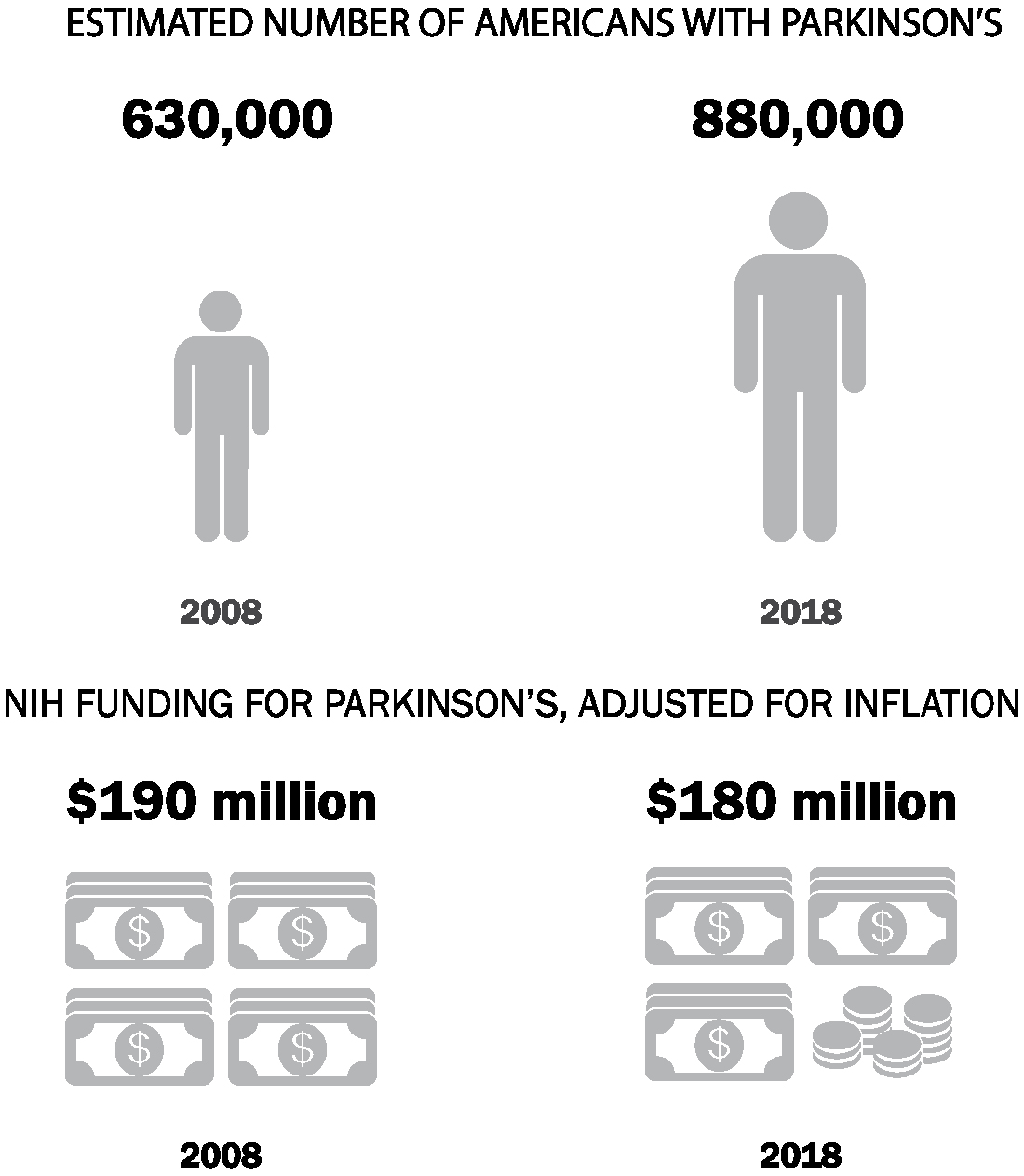
The NIH is the largest public funder of biomedical research in the world, spending $37 billion in 2018.26 However, its support for Parkinson’s research has waned over the past decade, just as the numbers of people with the disease have shot up (Figure 2). Adjusted for inflation, the NIH spent $192 million on Parkinson’s disease research in 2008. Ten years later, in 2018, it spent $177 million, or 8% less.27 At the same time, the estimated number of Americans with Parkinson’s increased 40%.28 Without advocacy, even well-intentioned organizations like the NIH can move in the wrong direction despite the tremendous increase in the burden of Parkinson’s disease.
The decline in US public support for Parkinson’s research has had an unlikely savior—a Canadian. In 1991, Michael J. Fox was a thirty-year-old film and TV star. While filming Doc Hollywood, he noticed a tremor in his left hand and that his arm wasn’t swinging normally when he walked; he was eventually diagnosed with Parkinson’s.30
Nine years after his diagnosis, he created The Michael J. Fox Foundation for Parkinson’s Research. Two years before that, in testimony to Congress, Fox said that he “was shocked and frustrated to learn the amount of funding for Parkinson’s research is so meager.”31 Since its establishment, the foundation has devoted $900 million to research programs, becoming the largest nonprofit funder of Parkinson’s research in the world. The organization has no endowment. It spends all the money that it raises in a given year. In 2018, it spent nearly $100 million on research (Figure 3).
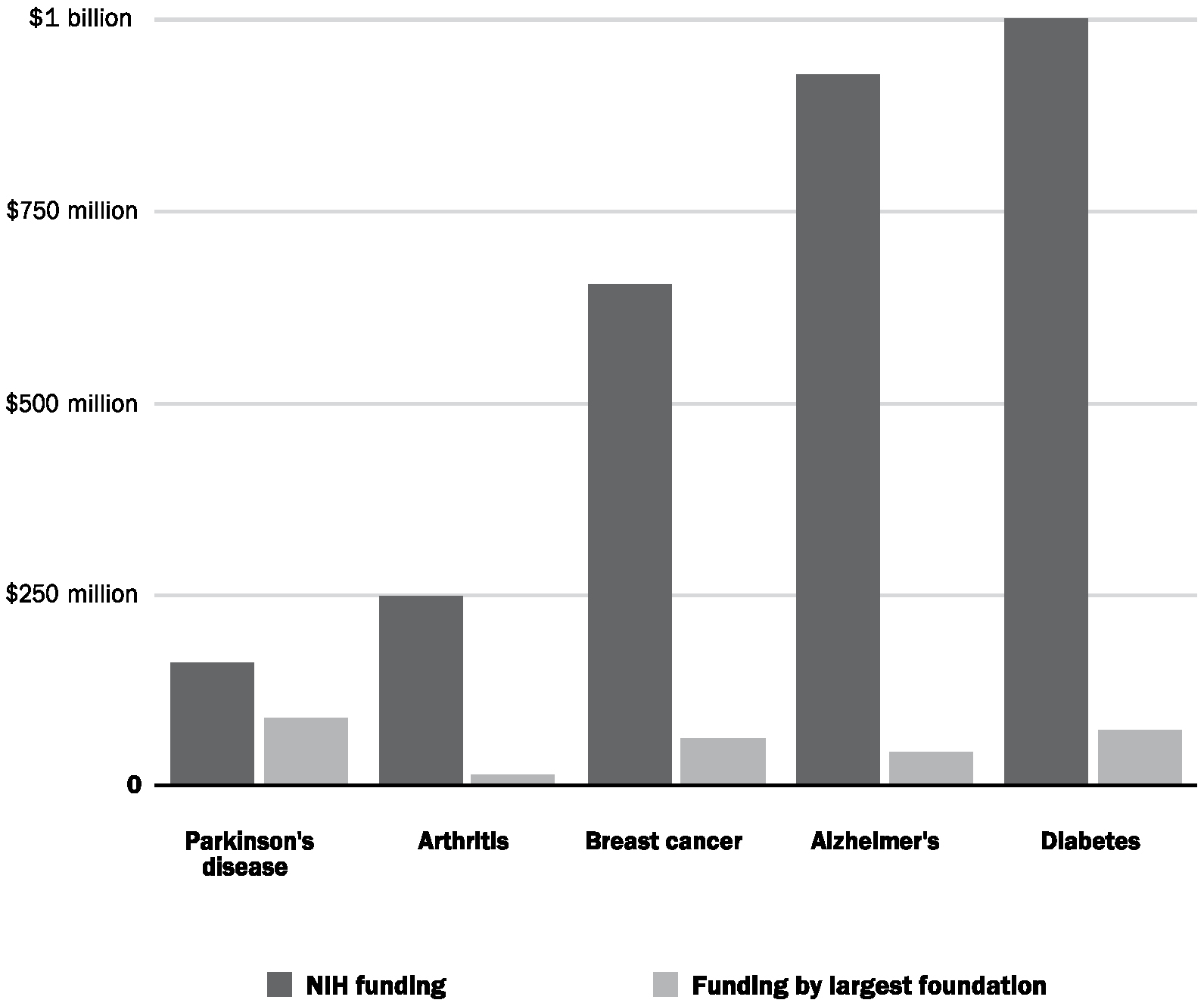
FIGURE 3. Comparison of NIH funding with research expenditures by the largest charitable foundations for select diseases, 2016.32
The foundation invests in studies and builds tools, such as genetic databases, for the research community to use. Its research portfolio spans basic research with rodents to clinical research involving humans. The foundation also funds therapeutic projects in partnership with universities and pharmaceutical companies, and its early-stage investment propelled inhaled levodopa to market in 2019.33 By bypassing the digestive system, the inhaled levodopa can provide faster relief of symptoms.34
UNDERSTANDING THE CAUSES OF PARKINSON’S DISEASE
The Michael J. Fox Foundation has a worthy goal: to cure Parkinson’s disease. This is the common hope for many groups fighting various illnesses. However, the greatest opportunity may be in preventing Parkinson’s in the first place.35
Dr. Caroline Tanner is the neurologist at the University of California, San Francisco, who has led numerous studies that have linked pesticides, TCE, and other environmental factors to Parkinson’s.36 Despite this groundbreaking work, she has not been able to do further large-scale studies aimed at identifying environmental risks. Why? “No one was willing to fund the research,” she said. Some efforts are beginning, but more are needed.37
To better understand environmental causes of Parkinson’s, simple maps that show the rates of the disease by geography would be a great start. These maps of “hot spots” overlaid with information about pesticide use, TCE contamination, air pollution, or other environmental factors could help scientists and others identify what might be causing the disease.
Researchers have maps of hot spots for cancer. Through a public NIH website, anyone—citizen or scientist—can find the rates of any cancer for any part of the country.38 Anyone can see where the rates of colon cancer, for example, are highest or lowest and what the risk is by age or race. We can also see what the trends are over the past five years.
For Parkinson’s there is very little of this helpful information. Studies that identify geographic variability, such as those showing that people who live in some rural areas have higher rates of the disease, make a strong case that environmental factors lead to the development of Parkinson’s.39 What we lack are details and real-time data. We cannot zoom in on any specific area and determine what the rates of Parkinson’s are or learn what pesticides or other chemicals have been or currently are being used. With rare exceptions, neither citizens nor researchers can determine the trends in the rates of Parkinson’s disease in their states.40
For a decade, Tanner and her colleagues have worked to develop such a map for California through a statewide registry of Parkinson’s.41 On July 1, 2018, the California Parkinson’s Disease Registry was finally launched.42 Previously, Nebraska and Utah were the only states to have such a registry.43 Data from the Nebraska registry allowed scientists to see that the rural, farming regions had two to four times more Parkinson’s than urban areas and that men had roughly twice the rates of the disease as women.44 Additional studies should quantify the risk posed by specific environmental risk factors and correlate those exposures with subsequent rates of the disease. And, of course, we need to be looking at every state—not just three.
Studies of Parkinson’s need to be large. Even though the number of people with the disease is rising, the total number is small relative to very common conditions such as diabetes. So in order to get reliable data and see trends clearly, large populations have to be studied for years. Such studies could track the rates of Parkinson’s among people living near TCE-contaminated Superfund sites. They could allow researchers to determine if certain groups of individuals, such as dry cleaners, are more likely to develop Parkinson’s.
These efforts should also be expanded globally. For example, identifying potential risk factors for the rapid rise of Parkinson’s disease in China is critical to slowing the disease.45 The risks from pesticides and air pollution could be quantified and compared to one another. Such data could inform what environmental actions should be taken and what short-term protections should be offered.
The genetic causes of Parkinson’s are also worth further investigation. Over the past two decades, Dr. Andy Singleton, who is now chief of the Laboratory of Neurogenetics at the NIH, has led pioneering genetic studies of the disease. Six years after researchers identified the first mutation in the alpha-synuclein gene, he and his colleagues identified another rare mutation that causes an early-onset, inherited form of Parkinson’s disease.46 One year later, he and his research team identified mutations in the LRRK2 gene that lead to Parkinson’s.47
Before the genetic causes of the disease were identified, few good scientific targets for new therapies were available to researchers. Now, based on the work of Singleton and Dr. Matthew Farrer from the University of Florida and Dr. John Hardy from University College London, and others, drug developers have something to go after, which represents a huge leap forward toward a therapy or a cure.48
Again, cancer offers a model. Treatment aimed at the underlying genetic causes of the disease is transforming care. Cancer treatments are increasingly based on the individual characteristics and often underlying genetic mutations responsible for the tumor. For example, in melanoma, one class of drugs is effective if the skin cancer has a certain genetic mutation. But in those cancers without the mutation, the drugs are unlikely to work and can cause harmful side effects.49 This personalized approach, where a drug is aimed at the underlying genetics of the disease, now applies to colon cancer, leukemia, lung cancer, and prostate cancer too.50
Now that we have identified relevant genetic causes, the same could happen for Parkinson’s.51 Armed with greater resources, scientists can identify more of these targets and learn how to neutralize them, as is already happening with the drugs in development to defang the threat of an LRRK2 or GBA mutation.
In 1991, Hyam Kramer’s mother was diagnosed with Parkinson’s disease. Kramer asked her neurologist if the disease could be inherited, and he was told no. Twenty-five years later, Kramer developed a tremor in his right hand and right leg and was subsequently diagnosed too. What his mother’s neurologist could not have known was that a mutation in the GBA gene was responsible for her Parkinson’s.52
Kramer’s mother, a former librarian, lived with Parkinson’s disease for twenty-two years. She was cared for by her husband, a former World War II veteran, who would visit her daily in the nursing home where she lived for the last twelve years of her life. She required a feeding tube, and at the end of her life, she could no longer recognize her son.
Watching a loved one decline is difficult enough, but Kramer was also (unknowingly) witnessing his own future. Normally optimistic and confident, Kramer, a professional fund-raiser, became depressed. Still, he signed up for a Michael J. Fox Foundation–funded study called the Parkinson’s Progression Markers Initiative.
To help identify objective measures of disease progression, The Michael J. Fox Foundation launched the study in 2010. This global initiative has enrolled nearly 1,400 participants at more than thirty research sites. Every six to twelve months, participants undergo detailed clinical assessments, imaging tests, and sampling of their blood and the spinal fluid surrounding their brain and spinal cord. The goal is to identify biological markers (akin to cholesterol for heart disease) that will track the progression of Parkinson’s disease.53 The data—available to scientists around the world—have been downloaded over 5 million times by research teams from over thirty countries.54 Unfortunately, while knowledge has advanced tremendously, no objective measure of disease progression has yet been identified.55
For the study, researchers were looking for Ashkenazi Jews with Parkinson’s, and Kramer fit the bill. He learned that he carried mutations in both copies of his GBA gene. (Humans have two copies of every gene—one from their mothers, one from their fathers.)
As a participant in the study, Kramer, who lives in Boston, Massachusetts, travels every six to twelve months to New Haven, Connecticut, to meet with researchers. During these visits, the scientists take various measurements so they can understand the course of Kramer’s disease. They are looking at markers in his blood and spinal fluid and are using sophisticated imaging tests to see how the disease progresses. As of April 2019, seventy-three individuals like Kramer with Parkinson’s disease due to a GBA mutation had enrolled in the global study.
Kramer has already participated in a clinical trial of a new drug and intends to participate in more. He hopes that his involvement in research studies and clinical trials will “shed light onto future treatments that [he] or someone like [him] can take advantage of.”
For Kramer, there has been a side benefit too. Helping the science move forward has brought back his optimism. Now fifty-eight, he recently married his longtime partner. He says that participating in the research has given him a sense of making a positive impact. “In some ways, the last three years have been the best three years of my life,” he says.
UNDERSTANDING HOW PARKINSON’S DEVELOPS
Identifying the environmental and genetic causes of Parkinson’s is an important first step. The next step is to determine how these factors lead to disease. Animal studies can help us determine how risk factors contribute to Parkinson’s. For example, in rats, TCE kills off dopamine-producing nerve cells in the substantia nigra region of the brain.56 Other parts of the rats’ brain are less damaged.57 But it is unclear exactly how TCE causes this apparent selective loss of nerve cells.58
When it comes to how genetic mutations cause Parkinson’s, we do not have the whole story either. We know that the alpha-synuclein protein helps transport packages of neurotransmitters, enabling communication among nerve cells. We also know that these proteins can be misfolded—likely as the result of a genetic mutation—which leads to Parkinson’s.59 However, the steps from misfolding to disease remain to be identified. The functions of the alpha-synuclein protein, including those outside the brain, have also not been defined.60
In addition, we do not have a full understanding of the LRRK2 gene and all its mutations.61 Researchers are still determining the various functions of the protein that the LRRK2 encodes, or instructs. The protein that takes its orders from the LRRK2 gene is large and likely interacts with the alpha-synuclein protein.62 The changes in the protein that result from the different mutations are even more of a mystery.
Basic research, with its exploratory nature, could help fill in some of these gaps. Unlike applied research, basic research has no predefined purpose. Rather, it helps us understand how nature works. Unfortunately, it is often overlooked, even though therapeutic advances rest on knowing how a disease—a change of nature—unfolds.
Over the last twenty-five years, pharmaceutical companies have invested less and less in basic research.63 In 1994, pharmaceutical companies spent over half (57%) of their research and development funds on this early-stage research.64 By 2017, that percentage had dropped to 16%.65 Consequently, the responsibility for this work has fallen to academic labs and research institutes that are heavily dependent upon federal support and private philanthropy.66 In the United States, most biomedical research (about 60%) is funded by pharmaceutical, biotechnology, and medical device firms. The upshot is that basic research is dependent on smaller sources of funding—the NIH and private foundations, which represent 27% and 4%, respectively, of the total research pie.67
Increasing our knowledge of the function of different genes is critical to understanding how the interaction of genetic and environmental factors can lead to Parkinson’s disease.68 Dr. Tim Greenamyre, who helped link pesticides to Parkinson’s, and his colleague Dr. Jason Cannon recently wrote, “Many researchers believe that gene-environmental interactions account for the majority of Parkinson disease cases.”69 They called for “[future] studies examining as many known genetic factors as possible.”70 These studies could inform us about how genetic factors influence the response to pesticides, chemicals, and other environmental risk factors.71
Finally, more research is needed to determine how various factors, including caffeine, diet, and exercise, decrease the risk of Parkinson’s. This information is critical because it can empower us to take advantage of behaviors that are available to most of us. Eating well and exercising carry many secondary health benefits, of course, and have been overlooked for too long.
DEVELOPING BETTER MEASURES OF PARKINSON’S DISEASE
The management guru Peter Drucker once said, “You can’t manage what you can’t measure.” Unfortunately for Parkinson’s, we don’t measure the disease well.
For heart disease, we have electrocardiograms, echocardiograms, angiograms, blood cholesterol levels, and blood pressure. For cancer, we have blood markers, imaging, and biopsies. For HIV, we can quantify the amount of virus in the blood and count the number of blood cells infected by the virus. For Parkinson’s, the principal tools of the twenty-first century are the same ones that Drs. James Parkinson, Jean-Martin Charcot, and William Gowers used in the nineteenth century—a patient’s history and a physical examination. In other words, we have almost no objective measures of the disease.
The history and exam can be misleading even in the best hands. Autopsies of people with diagnosed Parkinson’s reveal that their doctors had been wrong about their diagnoses 10% to 20% of the time.72 People thought to have Parkinson’s disease were shown to have had different parkinsonian disorders. Others had Alzheimer’s disease or tremors due to another cause.73 Unfortunately, over the last twenty-five years, doctors have not gotten better at diagnosing the disease.74
Just as diagnosis of the disease remains subjective and error prone, so too does the measurement of symptoms. Currently, the determination of the severity of Parkinson’s disease symptoms, such as difficulty walking, is based on clinical observations by experts. The most commonly used Parkinson’s measure is a rating scale that includes a “motor examination” by a clinician.75 The examination requires a neurologist or other trained investigator to rate multiple features of Parkinson’s disease, such as tremors and speed of movement, on a simple but crude 0-to-4 scale.
Not surprisingly, experts can vary in their assessment of how pronounced the movements are. One may rate a tremor a 2 (mild) while another may rate it a 1 (slight). Also, because the rating scale uses a 0-to-4 scale, it is insensitive to small changes in the disease. Small changes can matter over a short period when you want to know if a treatment is making someone better or worse. For example, a man’s weight can go from 184.8 pounds to 181.4 pounds—enough to see if a weight-loss program is really working. However, his tremor cannot go from 3.4 to 2.9—only from 3 to 2. So, with existing scales, it is easy to miss genuine but small improvements in Parkinson’s symptoms. Dr. Andy Grove, the activist engineer, referred to a Parkinson’s disease rating scale as “a piece of crap.”76
When traditional approaches are not working, sometimes voices from outside the field can offer more creative approaches and fresh hope. As a kid, Max Little was a mathematical whiz who, like many young boys, enjoyed video games. His twin passions fused when he developed digital sound algorithms for video games, which enable players to hear unique sounds like a dog barking or cars racing. He then earned a PhD in applied mathematics at the University of Oxford in England, where he created new mathematical rules and techniques for measuring voice.77
He and his colleagues then used these techniques to assess the voice of individuals with Parkinson’s, which is softened early in the disease.78 In 2012, the researchers showed that mathematical analysis of voice recordings could tell who had Parkinson’s and who did not.79 The results could even be used to predict disease severity.80
In 2012, Little gave a TED talk titled “A Test for Parkinson’s with a Phone Call.”81 In it, he explained how his test could be administered with any digital microphone. He also used the talk to launch a worldwide study, the Parkinson’s Voice Initiative, which, within hours, had recruited over 1,000 participants to leave recordings of their voice over the telephone. After just a few weeks, it had collected over 17,000 voices and remains the largest “citizen-led” scientific study of Parkinson’s disease.
Little and his colleagues went on to develop a smartphone application that can take additional objective measurements of symptoms. Using the phone’s embedded sensors, the app measures gait, balance, finger tapping (to assess speed of movements), and voice.82 In a pilot study, the app could differentiate individuals with Parkinson’s from those without the disease.83
In subsequent research, those same assessments derived from smartphone sensors generated a “mobile Parkinson disease score.” This score combined assessments of voice, tapping, and walking into a single continuous measure of disease severity. The scale goes from 0 (normal) to 100 (severe disease) and can be performed by almost anyone with a smartphone anywhere anytime.84
Inspired by the research of Little and others, Apple Inc. developed an open-source platform called ResearchKit for smartphone applications. The platform enables anyone to build research studies on smartphones for any condition from autism to Alzheimer’s disease. On March 9, 2015, Jeff Williams, the current chief operating officer of Apple, announced to a packed audience and about 1 million people watching online the launch of five smartphone research studies, including one for Parkinson’s disease called mPower (Figure 4).85
In his talk, Williams identified several barriers to medical research—participation restricted to people who live near research sites, subjective data, and infrequent assessments—all of which the mPower app eliminates. With it, individuals can use their iPhones to enroll in the study, conduct objective assessments frequently, and receive their results back in real time.86 They can see their individual voice or tapping scores as soon as they complete the tests. In most studies participants only learn the results after the research is completed. Some never learn them at all.87
Williams gave his talk in the morning. By dinner, more than 2,000 individuals from across the country had enrolled in the Parkinson’s study. By contrast, the largest Parkinson’s disease clinical trial of a drug took three years to enroll 1,741 participants.88 Combining the Parkinson’s Voice Initiative’s approach of citizen-led recruitment with smartphones created a new paradigm for research studies.89
The science continues to accelerate. In 2017, a smartwatch study was launched to screen for abnormal heart rhythms and enrolled over 400,000 people in one year.90 In 2018, Apple publicly announced that the Apple Watch could also track symptoms of Parkinson’s disease.91 That same year, Verily Life Sciences (part of Alphabet, the parent company of Google) partnered with Radboud University Medical Center in the Netherlands and with The Michael J. Fox Foundation to investigate the ability of a watch to assess tremors, activity, and sleep.92 The initial results on adoption are encouraging—hundreds of Dutch patients are wearing the Verily smartwatch for twenty-two hours per day; some have done so for more than a year. Such observations allow insights into how people function in their own environments, which can differ from their performance in artificial clinical settings.
Objective measurement of the effects of new therapies is critical. Current subjective rating scales can lead to ambiguous results. For example, two separate studies of the same drug for Parkinson’s that were conducted with a nearly identical design and outcome measures had completely different results.93 Imprecise measurements have real consequences—they make it difficult to determine whether a new treatment works for Parkinson’s. As a result, clinical trials for the disease and similar disorders end up having to be larger, longer, more expensive, and riskier than for other conditions.94
Because of the risk and cost of clinical trials in conditions like Parkinson’s, large pharmaceutical companies may be reluctant to invest in them. On January 8, 2018, Pfizer announced that it was ending research into new drugs for Alzheimer’s and Parkinson’s.95 High-profile failures to find an effective drug for Alzheimer’s and the absence of substantial therapeutic progress in Parkinson’s disease likely contributed to the decision.96
Smaller companies are filling the void left by larger companies. In fact, about one year after Pfizer’s exit, it formed a spin-off company called Cerevel Therapeutics. Backed by $350 million in venture funding, Cerevel Therapeutics will develop new neurological drugs, including a promising one for Parkinson’s disease that acts on dopamine.97 The new company hopes in 2019 to begin a late-stage clinical trial of the drug, which may not have levodopa’s side effects.98
Many pharmaceutical companies are embracing digital measures for Parkinson’s. Roche used a smartphone application in an early-stage trial for Parkinson’s and found it to be more sensitive than traditional rating scales.99 It is now in use in a subsequent clinical trial. Other pharmaceutical companies are evaluating watches and other sensors with the plan to use them to assess future treatments.100
Cerevel and others will need these and other measures to gain an accurate assessment of whether their drugs work. As Little said, “The only way we’re going to know when we actually have a cure is when we have an objective measure that can answer that for sure.”101
OVERCOMING APATHY
In a cruel irony, helping end Parkinson’s depends on what the disease often steals—energy and a drive to make things happen. Apathy—a loss of initiative and blunted emotions—is often part of the disease.102 The late Leonore Gordon, a former family therapist and resident-poet in the New York City Public Schools, struggled with apathy during her twenty-year fight with Parkinson’s.103 But Gordon ultimately won.
She fought with activism. She figured out that helping others reduced her feelings of indifference. She gave talks, wrote poems, raised funds, and counseled hundreds. To help build awareness of how Parkinson’s could pull people down, Gordon participated in an educational television episode on apathy for the Parkinson’s disease community. On the show, she said that when people with the disease “get involved in advocacy or volunteering or being involved with anything where they feel needed, we get out of the house.” She also said, snapping her fingers, “When I get on the computer and see that someone needs me for help, apathy is gone like that.”104
Apathy and complacency are dangerous—beyond the dispiritedness that they bring. Our lifetime risk of dying in a car accident is one in one hundred. Consequently, almost all of us wear seat belts, most of us drive cars with air bags, and all of us want children to drive safely.105 Our lifetime risk of developing Parkinson’s is much greater—one in fifteen.106 And yet, most of us do little.
Like Gordon, we need to overcome our apathy. Unfortunately, Gordon died in 2018. In her memory, her friends wrote, “She was a warrior whose heart was full of love, who had the courage to speak out, to take action, and to motivate others to join her for worthy causes.”107 Let’s continue her efforts.