

Coroner’s Report
December 5, 1918
This twenty-two-year-old white male was in excellent health before falling ill.
Clinical History
Circumstances leading to death of patient
• Returned with troops from the Great War on steamer ship after the Armistice celebration in France, arriving home November 28, 1918. Admitted to military infirmary on November 30, complaining of mild flu symptoms: head and body aches, sore throat, dizziness, poor appetite, and fever of 39.7°C (103.5°F).
• Patient transferred to hospital. Symptoms developed: drowsy, lethargic, and with low pulse. Bed rest and fluids prescribed by attending doctors. Patient developed a dry cough, but perked up briefly when his temperature returned to normal.
• Sudden relapse on December 2. Fever spiked to 40°C (104°F). Increased cough with blood in mucus. Breathing raspy and patient frequently choked. Died about 11:00 pm.
General Findings
Observations of the exterior of the body
• Skin, especially on face, cyanosed—purplish blue and blotched.
• Foamy mucus in nose and mouth cavities contained dried blood.
• Frequent sneezing and nose-blowing appear to have caused redness of skin around nose and chapped lips.
Observations inside of the body
• Patient constipated—evidence of dehydration.
• Lungs show signs of advanced pneumonia: pus-filled lobes speckled with burst blood vessels.
• Lungs have the consistency of soft blackberry jam. Normal uptake of oxygen would have been severely impaired.
• Toxemia: bacteria from the lungs overwhelmed the bloodstream with infection.
Conclusions
The patient died from a severe case of influenza—likely Spanish flu—possibly acquiring the viral infection during the end-of-war celebrations or in close quarters on board ship. Cause of death was asphyxia, choking on body fluids coughed up from his lungs. He was highly infectious at the time of death. Those who traveled, roomed, and ate with him in the days before he became sick, as well as those who cared for him during his illness should be advised to watch for flu symptoms.
In the last year of World War I, the worst flu pandemic in history traveled the globe, cutting down more people than were killed in the war itself. Estimates range widely, from twenty-one to sixty million people.
Unlike “normal” seasonal flu, which usually targets the very young and old, the 1918 pandemic also overwhelmed people in their twenties and thirties, the fittest and healthiest. And because soldiers gathered in large groups on military bases and boats, in prisoner-of-war camps, and in the trenches, they were sitting ducks for catching flu.
This flu pandemic was unusual—including the name. Why was it called the Spanish flu? Spain was hit hard by the flu. And the rest of the world heard about it through the Spanish media, hence the name.
Conditions of war—such as rationed food, cold homes due to lack of heating fuel, and a stretched-thin health-care system—also gave this nasty flu virus the advantage. The sheer number of deaths created shortages in coffins, undertakers, and gravediggers.
Spain was a neutral country and therefore didn’t hide the impact of the flu and the number of deaths. But the countries engaged in combat lowered a veil of secrecy around the numbers of their sick and dead. Neither side wanted to admit that their soldiers were weakened.
Rumors circulated that this pestilence was actually a German biological weapon. But then the flu reached Germany, too. In fact, the Spanish flu may have turned the tide in favor of the Allies against the Axis powers. A planned assault on England was cancelled because so many front-line German soldiers lay sick, dying, or dead.

Even if politicians and generals had admitted there was a pandemic, we can’t be certain much would have changed. Scientists were unable to find the source of the disease and could not develop an effective vaccine. Because this flu struck in the days before electron microscopes, scientists literally couldn’t see what they were up against. This particular influenza virus was so small that twenty thousand viral particles could sit on the head of a pin.
Today, because virologists and microbiologists think another pandemic looms, there is still urgent curiosity about the Spanish flu. Scientists hope that by studying specimens reclaimed from old laboratory slides and exhumed bodies they can determine why the Spanish flu was so deadly. They hope that an understanding of how the Spanish flu behaved will help prepare for, or prevent, the next pandemic flu.
For many years, a cook in a Kansas military camp was thought to be the first confirmed Spanish flu case in 1918. He traveled to Europe on board a ship and took the flu with him. But the work of virologists such as Professor John Oxford of England challenged this theory and suggested the cook got sick at the start of a second wave of Spanish flu on European soil. Oxford found that an identical flu did the rounds at a military base in France in 1916 and concluded the flu of 1918 was so intense because it had mutated and become stronger.
“Step on a crack, break your mother’s back. Step on a line, break her spine.” In the early 1960s, Penny told her friend Janie (now Jane Drake, one of the authors of this book) that this was true—and Janie believed her. Janie chanted the rhyme and skipped down the sidewalk, aiming for smooth pavement, until she bounded up Penny’s steps. A huge sign posted by the Medical Officer of Health hung on the front door:
SCARLET FEVER
These premises are under
The Department of Public Health
QUARANTINE
The window on the top floor creaked open and Penny poked her head out and waved. No one could go in, and she couldn’t come out. Penny’s sister had scarlet fever and the entire family was trapped inside for a week, even the dog. Janie had lots of questions. Stomping home, she forgot about the cracks and lines. This was no time for superstition.
Fifty years ago, when your grandparents were kids, contagious diseases such as scarlet fever routinely infected whole families, spread like wildfire through neighborhoods, and shut down schools. Kids frequently developed serious health complications or even died.
Before antibiotics, people were frightened by a diagnosis of scarlet fever (an infection from the same bacterium that causes strep throat). Most kids who fell ill with this sickness would feel miserable for several weeks, suffering through a sore throat, pus-covered tonsils, peeling skin, chills, vomiting, aches, and pains. A red, sandpaper-like rash would appear, lingering for two or three weeks as a reminder of the “scarlet” in the name.
Most sufferers would get better. But in some cases, the infection would camp out in the ears, sinuses, or chest, making recovery slow and painful. Some suffered pneumonia, meningitis, or kidney or liver problems—leading to longer illness or death.
For unknown reasons, the scarlet fever bacterium gradually weakened and no longer packed the same punch. These days, outbreaks are rare thanks to antibiotics and alert public health workers, but they still occur.
MMR is an acronym for a combined vaccine for measles, mumps, and rubella. It has saved millions of lives, reduced unnecessary sickness, and prevented uncountable missed school days. Anyone under forty-five years old was probably vaccinated as a kid.

Some grownups may have sentimental memories of being home sick with measles—Popsicles, ginger ale, cooling chamomile lotion, one-on-one time with Mom, card games, daytime TV—but it remains a serious global illness with twenty million cases a year.
The World Health Organization (WHO) lists measles in the top five diseases that kill children under five—eighteen died every hour in 2008. But this highly contagious virus is preventable by vaccine.
After an incubation period of over a week, sufferers begin to show obvious signs of sickness. The most distinct symptom is a rash that starts on the head and travels down the body over the next week. Sometimes the rash can appear inside the mouth—small red spots with blue-white centers. These dots are called Koplik’s spots, named after Henry Koplik, who was the first to document them in 1896.
Some people who contract measles get horrific complications: dehydrating diarrhea, encephalitis (swelling of the brain), or pneumonia. On rare occasions, a sufferer who recovers is left deaf, blind, or mentally disabled. It’s no wonder that WHO, UNICEF, and others are taking the lead in eliminating measles worldwide. In the last ten years, these organizations have immunized millions of kids in Africa and Asia—and the infection rate is plummeting.
But measles can still strike in North America. In fact, the headline “Public Health issues warning about measles exposure in downtown Woodstock store” appeared in a small town newspaper in Ontario, Canada, in October 2010. How could this be possible?
It turns out, a visitor from another country, someone who had never been vaccinated, was infected with the highly contagious virus and walked into a decorating store in Woodstock. The threat of infection remained two hours after the sufferer left.
Anyone who had been in the store during that time could have contracted the illness if they hadn’t been vaccinated yet, were never vaccinated, or had been improperly vaccinated. Those people who thought they might have been exposed were warned to watch for the classic measles rash.
Before widespread vaccination, Canada reported about three hundred thousand cases of measles every year, with five thousand treated in hospital and several hundred deaths. Since 1998 in Britain, some parents have refused to vaccinate their children because they have been misinformed about the risks of the process. Now there are several thousand cases in Britain every year. A spokesperson for the Department of Public Health wrote, “Until measles is eliminated from the planet like smallpox was, vaccination will continue to be the most important strategy to protect people against this disease.”
The middle M in MMR stands for mumps. Ann Love, one of the authors of this book, remembers her brother asking at dinner, “What’s the matter with your neck?” He used his fork to poke toward her. He was usually a pest, but this time he was right. Her face felt full and she was having trouble swallowing her mashed potatoes.
Mumps was going around Toronto in the spring of 1958, and Ann must have breathed in viral droplets from one of her classmate’s laughs, sneezes, or coughs. In fact, whoever gave it to her might not have seemed sick.
But Ann got a bad dose of the illness and soon spiked a high fever, lost her appetite, and the glands at the back of her mouth—responsible for making saliva—swelled and ached. Her brother called her Chippie, short for chipmunk, but she was too sick to care.
If she passed along the sickness to him, his testicles might swell and then he wouldn’t think it was funny. Other nasty complications could include convulsions and inflammation of the brain and spinal cord. She hoped he wouldn’t get it.
Mumps is no longer a menace of childhood. An effective vaccine was developed and introduced in his country by the American microbiologist Maurice Hilleman, in 1967. Today, kids with up-to-date vaccinations don’t get mumps. And that’s a good thing, because there is no treatment except ice packs and painkillers!
Otherwise known as German measles, rubella (the R in MMR) is an illness that is milder than measles, but it can have tragic consequences. Pregnant women exposed to the virus who are not immune (meaning that they’ve never had the disease and were not vaccinated) can miscarry or give birth to a child with severe disabilities. The infection starts out with cold- or flu-like symptoms and a rash appears a day or two later.
Whoop, whoop, gasp, gag! Where’s the bucket? Vomit. Whooping cough, or pertussis, also starts out like a cold—runny nose, tickle in the throat, and a low-grade fever. Then the diarrhea starts. Finally, the sufferer endures deep spasms in the chest and a cough so wrenching that the capillaries in the eyes can burst, making the white part bright red. When you’ve got whooping cough, you’re in for six weeks of misery.
Whooping cough gets its name from the sound the patient makes when he or she finally takes a deep breath after a coughing jag. Cough medicine can’t touch this bacterial infection. Without antibiotics, whooping cough is contagious for at least two weeks.
Normally, doctors say twenty-four hours on antibiotics stops contagious infection, but with whooping cough, it takes five days. It’s no wonder whooping cough can quickly turn into an epidemic. The World Health Organization (WHO) reported that sixteen million people had whooping cough in 2008 and 195,000 children died. When will the last whoop be heard? Not until everyone is vaccinated and whooping cough has no one to infect.
Start with a highly infectious bacteria or virus. Add a rash, fever, headache, and cough—and you could have any number of childhood diseases. Over time, we’ve learned that prevention is better than cure.
After lunch, I sat at my desk and wondered whether I’d eaten something rotten. Heat flushed my neck and face before cramping pain grabbed my middle. Several times during the ride home, I opened the passenger door and threw up on the road. All I could think about was bed. If only I could curl up in a ball and sleep.
But the pain became more intense—had an alien taken hold in my belly? Finally, my family rushed me to hospital. In the ER, a young doctor palpated my abdomen. Her cool hands pressed down slowly, then she let go quickly. As my flesh bounced back into place, the point of agony focused below and to the right of my bellybutton. Wheeling me to the operating room, the nurse asked the doctor if the appendix had ruptured.
This book might not have been written without antibiotics. Both authors of this book had ruptured appendixes that were surgically removed, followed by antibiotics.
The appendix is a little organ attached to the intestine. Doctors can’t agree on its function and many believe it’s actually unneeded in the human body.
Regardless of the debate, we do know that when the appendix gets infected and bursts, the belly fills with pus. The pain is excruciating. Without antibiotics, the infection can fester, spread, and kill the patient.
Consider your own childhood illnesses: you’ve probably taken antibiotics for an infected throat, ear, tooth, or cut. In the days before antibiotics, any of these common complaints could have led to prolonged sickness, gross complications, and death. The antibiotic penicillin is probably the most important drug discovered so far.
Alexander Fleming, the microbiologist who discovered penicillin, wasn’t always a science nerd. Born into a large Scottish farming family in 1881, he attended a small village school before moving to London, England, in 1895. He had no burning desire to be a scientist. In fact, after two years of college, no particular area of study grabbed his imagination and he took a job as a shipping clerk.
Bored after four years of work, he used a small inheritance to pay for medical school tuition. One summer in between terms, Fleming took a job in the laboratory of vaccine pioneer Almroth Wright. His experiences there lit a fire under him, turning him on to bacteriology and immunology. Despite graduating as a surgeon, Fleming’s fascination with infectious disease pulled him back into the laboratory where he happily spent much of his career hunched over a microscope. A nerd at last!
Fleming spent World War I in frontline battlefield hospitals along with his colleagues from Wright’s lab. He saw firsthand the horrific effects of bacterial infections that rotted off the body parts of soldiers wounded in trench warfare. He became convinced that a nontoxic antiseptic could be used as a powerful weapon against infection.
Fleming and his colleagues compared the injuries of soldiers. They observed that wounds washed out with standard antiseptics healed more poorly than raw, fresh wounds left to heal on their own. Fleming’s group studied the blood and clear liquids that oozed from fresh wounds and discovered that they contained phagocytes, the white blood cells that gobble bacteria.
Fleming realized that the body’s own healing powers—the white blood cells—were neutralized by the antiseptics. He tried, unsuccessfully, to persuade field hospital doctors to cleanse wounds with a nontoxic salt solution instead.
Fleming didn’t think he was extraordinary. He believed that new ideas—and sometimes scientific discoveries—came from following hunches, sticking with a problem, and challenging himself. Back in his London laboratory after the war, Fleming studied all forms of secretions—including mucus, tears, pus, and blood serum. He asked his family and friends to give him their sneezed-into tissues—and other specimens—for his endless experiments. Fleming discovered that the body produces its own natural antiseptic—an enzyme that breaks down the walls of many bad bacteria. Found in saliva, tears, and other body liquids, Fleming called this antiseptic lysozyme. Fleming’s plea to use it, rather than wash it away with strong cleansers, was a step forward in understanding wounds.

Fleming’s laboratory was not a neat place. But it’s this lack of tidiness that led to his most important discovery. Returning from a holiday, he found his petri dishes of staphylococci bacteria cultures—part of an experiment he had been conducting—were all overgrown, except one. In this dish, there was only mold growing where there had once been bacteria.
A tidier and less observant person would have dumped out all these dishes, disappointed in a spoiled experiment. But Fleming took a closer look and slowly got excited about his discovery of a mold that could kill bacteria. He first nicknamed it mold juice and later called it penicillin. “When I woke up just after dawn on September 28, 1928, I certainly didn’t plan to revolutionize all medicine by discovering the world’s first antibiotic, or bacteria killer,” he said. “But I suppose that was exactly what I did.”
Fleming spent the next ten years trying to produce enough mold juice to keep up with his experiments, but he became discouraged when drug companies showed no interest in his work.
Howard Florey and Ernst Boris Chain, Fleming’s colleagues from Oxford University, kept the promise of penicillin alive, proving it cured infections in mice. Penicillin became available to wounded soldiers halfway through World War II. This new miracle drug meant fewer soldiers lost limbs or died from infection. Sir Alexander Fleming, Sir Howard Florey, and Sir Ernst Boris Chain shared the Nobel Prize for medicine in 1945.
The warm weather came early in 1937, when a terrible polio epidemic hit Toronto, Canada. Several kids in our neighborhood disappeared into the Hospital for Sick Children and I can still feel the panic. Daily newspaper headlines cautioned parents to keep their children home, but my mother, Sadie Barnett, took that warning several steps farther. We were strictly forbidden to play with our friends and banned from answering the door, and hand-washing was frequent and thorough. My parents whispered late into the night, and we seven children had our ears to the wall.
One evening, Father announced we were leaving for the country—before school was out for the summer holidays—and we’d stay until after the first frost. Our excitement was squashed by his flash of anger as he reminded us we were more fortunate than others, having a safe place to go.
These were the late days of the Great Depression—before Canada had health care for everyone. Parents couldn’t afford doctor bills, so they waited to see if their child really had polio before seeking medical attention. But the symptoms were vague and flu-like—queasy stomach, headache, sore muscles, and so on.
Not much was known about the disease then, but one thing was certain. In some cases, kids became paralyzed, their limbs withered. Or sometimes their throats seized up and they couldn’t swallow or even breathe properly. We knew that you were most likely to get polio if you were young—like me and my siblings.
That summer at the cottage was like living in a parallel universe. Most days, I walked a quiet country road to the nearest village and bought a Toronto newspaper. I read that pools and parks had closed and that kids weren’t allowed in theaters or even churches.
And the authorities were threatening to close down the Canadian National Exhibition on Children’s Day—a rite of childhood at Toronto’s annual fair. I went swimming in the lake every afternoon and enjoyed the outdoors with my brothers and sisters, glad that my parents had decided on our family quarantine.
There was no cure for polio, and the paper was full of debates among medical scientists. Some favored a liquid called convalescent serum (a serum obtained from a person who has recovered from the infection already, like a vaccine). Others believed that a nasal spray could prevent the virus from entering the body through the nose.
Eventually, both medicines proved useless. What did seem to help was a respirator called an iron lung. The Hospital for Sick Children had one such machine, and in August of that summer, it saved the life of a young girl whose lungs were paralyzed by polio.
When another child was admitted needing an iron lung, they built another “lung” using a respirator for premature babies and a wooden box. Nicknamed the lumber lung, it did the trick. The results were so dramatic that more were made in the hospital basement.

Around that time, I developed a headache, fever, and a painful, stiff neck. I hid my symptoms from Mother because she’d “freak out,” as my grandchildren would say now, and make me stay in bed. It was hot and I wanted to swim with my brother Victor, so I kept quiet and felt better in a few days.
It wasn’t until I was in medical school five years later that I learned that most people who got sick during the epidemic didn’t know they had the disease. They made complete recoveries. With the help of my medical school classmates, I found physical reminders of my bout with polio. I have one shriveled muscle in the back of my neck and a weak muscle in one upper arm. That’s probably why I never made the baseball team in high school.
—Henry Barnett
To learn more about polio, the authors of this book interviewed their dad, Henry, about his personal experience with a polio epidemic. Now that he’s ninety, Henry looks back and realizes he was very lucky—especially compared to his friend and classmate Hugh MacMillan, who, thirteen years later at the age of twenty-nine, was struck hard with polio.
Dr. Hugh MacMillan’s wife was hospitalized with polio in 1950, and Hugh was a faithful visitor—until he too came down with the disease. She made a complete recovery, but he became paralyzed.
Unable to move from the neck down, Hugh required an iron lung machine. But gritty determination and extensive therapy brought back some movement and he eventually spent time out of the iron lung with the help of a special rocking chair and a technique—called frog breathing—of gulping down air.
With support from his family and friends, he invented devices that kept him independent and as productive as possible, including tools that enabled him to play cards, make phone calls, and feed himself. In the last few years of his life, he worked at the Ontario Crippled Children’s Centre where he was a fantastic role model for disabled youngsters. Although he died in 1964, his example provides inspiration for many physically disabled people today.
President Franklin D. Roosevelt, a paralyzed polio victim since 1921, founded the National Foundation for Infantile Paralysis (NFIP) in 1938. From the beginning, the NFIP held an annual fundraising campaign nicknamed the March of Dimes after a song popular during the Great Depression called “Buddy, Can You Spare A Dime?” North Americans generously donated pocket change.
In 1947, the foundation hired Jonas Salk, an American medical doctor who specialized in virus research. Devoting his efforts to polio, Salk’s work helped isolate the three kinds of polio in an effort to eventually develop a vaccine. Called the “summer plague,” because the disease usually struck in the heat of the summer, polio continued to terrorize North America. The worst polio outbreak occurred in 1952, with fifty-eight thousand cases reported in the United States alone.
That same year, Salk’s laboratory made a vaccine from killed polio virus. He tested it on children who already had polio before giving it to himself, his wife, his children, and volunteers. The vaccine, proven safe and effective, was then administered nationally to over a million kids—with amazing results.
In 1957, the March of Dimes sponsored a campaign to immunize all children in America, and the number of cases dropped to 5,600 that year and down to 121 by 1964. It became clear that polio could be beaten. Salk did not patent his discovery, hoping a low-cost vaccine would reach more people.
In 2011, WHO, UNICEF, Rotary International, and the Bill and Melinda Gates Foundation (Microsoft’s founder) spearheaded the final push to rid the world of polio. Africa and India still have outbreaks, but Afghanistan and Pakistan are the only countries where new polio patients are regularly diagnosed.
India is using 2.3 million volunteer vaccinators to reach the most isolated and impoverished communities. Carrying the precious liquid in coolers and wearing bright yellow vests, volunteers approach mothers and their babies wherever they find them—in railway stations, markets, and on the street. Once a child is vaccinated, his or her fingernail is marked with permanent marker.
Dad found Tyler in his bedroom. He flicked on the light, asking, “How did the soccer tournament go?”
His son groaned and snarled, “Turn off the light. It hurts my eyes.”
The room stank of vomit and the boy’s forehead was scorching hot.
Dad peppered him with questions. “When did this start? Where does it hurt? Are any of your teammates sick?”
Tyler’s voice was slurred as he murmured details of a headache and wanting to sleep.
Dad’s worry turned to chilling fear when he focused on Tyler’s foot, which was flung outside the bed sheets. The skin on his ankle and toes was purple, blotched, and swollen tight. Dad’s brain screamed, “Call 911. This is a medical emergency!”
Tyler was losing consciousness by the time the ambulance arrived. Jake, the paramedic, called ahead to the ER and then slickly jabbed Tyler’s thigh with an intramuscular injection of antibiotics. He started the IV as Tyler’s pulse dipped dangerously low. Tyler was headed for septic shock.
With this strain of meningitis, minutes count and the ER team cranked into high gear. First the ABCs: airway clear, breathing shallow and rapid, circulation in distress. Start the IV fluids pronto and inject the first dose of steroids to reduce brain swelling. Give more antibiotics—try and beat the infection before it kills the skin and underlying tissues. Heart and blood pressure monitors …
In just a few hours, Tyler had transformed from a healthy eighteen-year-old into a critically ill patient in the intensive care unit. What he’d thought was the beginning of flu was actually meningococcal (bacterial) meningitis. It probably started when he borrowed a teammate’s water bottle the day before.
He would spend two weeks in the hospital, lose parts of two toes on his left foot, and be scarred by raised and twisted markings on his ankle and leg. His recovery was slow and painful, but he considered himself lucky. He wasn’t brain damaged—he could see and hear. And soccer was still his game.
Anyone can get meningitis, but it’s most likely to infect children under a year old in settings such as daycares and nursery schools, or young adults gathered together in army barracks, camping quarters, school cafeterias, and dorms. In Tyler’s case, kids traveling as a team—sharing a bus in close quarters, grabbing a water bottle when thirsty—set the stage for an outbreak of meningitis.
It’s a mystery why some people get sick while others don’t. If someone does get sick, the best public health response is for family members and others in close contact to immediately take antibiotics. Vaccines prevent some of the severest strains of meningitis, and some colleges and camps even require their visitors to be vaccinated before attending. If Tyler had it to do over …
Meningitis is caused by inflamed meninges—the sensitive membranes protecting the outside of the brain and spinal cord. Although sometimes caused by a fungus or traumatic brain injury, or as a complication from surgery, meningitis usually starts with an ordinary infection. The microbes travel through the bloodstream, ending up in the meninges. Meningitis takes hold and rapidly transforms into a different, much nastier illness.
Viral meningitis is the most common form of the disease. Usually, people fully recover without long-term side effects. Because it’s a virus, antibiotics don’t work. Instead, patients need rest, fluids, and painkillers for about ten days.
The bacterial form of meningitis is vicious but rare. It strikes hard and fast—sometimes killing its victim in a single day. Bacterial meningitis kills up to one in ten infected people. Untreated, it is fatal half the time.
Both types of meningitis start with the same symptoms: stiff neck, vomiting, fever, extreme crankiness, no energy, loss of appetite, and a headache that is sudden and sharp. Getting the right diagnosis and care at this point is critical. Doctors might start antibiotics before the test results are in, alarmed by the downward spiral of the sick patient. And the drugs can stop the savage destruction that bacterial meningitis inflicts on the body and even save life itself.
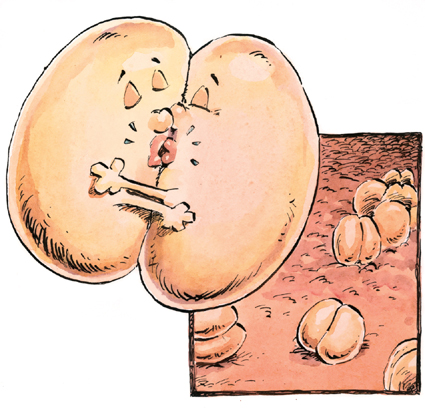
What do two kissing kidney beans have to do with meningitis? Medical students learn about hundreds of diseases and use tricks to remember them. Under the microscope, the bacterium that causes meningococcal meningitis looks like two kidney beans facing each other and sharing a kiss, their “bodies” forming a small doughnut shape. And you can get meningitis from kissing or from being in very close contact with someone who has the illness. Now you won’t forget, either.
Like a perfect storm, meningitis has found the world’s weak spot—it stretches from Ethiopia in the east to Senegal and Gambia in the west. This meningitis belt, as it is sometimes called, is a dusty, windswept African region that is in constant upheaval from war, famine, and poverty. It is home to the world’s biggest concentration of meningitis outbreaks, epidemics, and deaths.
With the financial backing of the Gates Foundation, the Meningitis Vaccine Program aims to stamp out meningitis epidemics in Africa. In its first year (2010–11), twenty million people received injections of a vaccine developed with Gates’s money. Each dose cost fifty cents.
Curious children kibitz on the wharf as the boat’s engine cuts and passengers hop ashore. This remote island on Lake Victoria, Tanzania, doesn’t get many visitors, but these ones know the drill. The children and a large group of other villagers escort the small contingent of international health workers to the chief’s hut.
He’s getting creaky with age and no longer walks down to meet outsiders. Hugging and clasping hands show respect and friendship, and sharing tea is a welcome activity on a dusty, hot day. Through an interpreter, they chat about weather, livestock, and the chief’s grandson’s wooden carvings—keeping things light.
Finally, the chief points out three of his people who need medical attention. One health worker produces the testing kits and demonstrates in mime how to swab one end along the soft tissue on the inside of the cheek until it’s coated with saliva. The three testing swabs are laid out on a table and conversation resumes for an agonizing twenty minutes. A health worker examines the swabs one by one, and the results are clear on his smiling face. All three test negative for HIV.

Now the doctor can check for another cause for the local outbreak of diarrhea and weight loss—malnutrition, hookworm, yellow fever, or malaria—and hopefully help them heal. Nothing positive comes from being HIV positive.
In 1980, a new illness first known as the slim disease was reported around the Lake Victoria region of Eastern Africa, which includes Kenya, Uganda, and Tanzania. About the same time, otherwise healthy young men from the gay community in San Francisco and New York were seeking medical care for an alarming new disease. Researchers in the United States and France soon identified HIV—a unique and incurable disease.
The acronym HIV stands for human immunodeficiency virus. We can learn details about the disease by looking at the words that make up the acronym. It is a disease that is specific to humans (H), one that attacks the body’s immune system (I), and it is a rapidly changing virus (V)—not a bacterium. The immune system is the very system designed to fight infectious illness, so once this disease takes hold, victims become more vulnerable to other illnesses.
AIDS, the second and terminal stage of HIV, stands for acquired immune deficiency syndrome. Again, look at the words in the acronym. The disease is not hereditary, meaning that it doesn’t run in families. Rather, it is acquired (A). Immune deficiency (I and D) relate to how the disease strikes the cells and organs that keep the body well. Finally, AIDS is a classified as a syndrome (S) because it is a complex combination of symptoms, not a single disease.
Like many other serious illnesses, HIV starts out like the flu, lasting three or four weeks. Sometimes, those infected will experience a lull, or latent phase, of several months or as long as ten years. During this time, individuals are infectious, but many don’t show symptoms even though their immune systems are slowly failing.
The immune system has several layers of defense. On the outside, skin acts as a protective barrier. Inside the body, in the bloodstream, phagocytes gorge on infectious microbes while infection-fighting chemicals are released when the immune system calls for help. The strongest and most specific defenders in the blood are the T cells and B cells, which are white cells produced in bone marrow.
When HIV gets in the body, it starts replicating itself by invading and killing white cells, specifically the CD4+ T helper cells. When healthy, these helper cells check out all the other cells, making sure they are normal. When they discover abnormal cells, CD4+ T cells activate other cells designed to kill infections.
HIV gradually kills off so many helper cells that the body can’t activate its own defenses. At this point, HIV is called AIDS, and the patient usually dies of a secondary infection such as tuberculosis, pneumonia, or one of several types of cancer such as Kaposi’s sarcoma or non-Hodgkin’s lymphoma.
Since 1981, researchers have scrambled for answers to three big questions: Where did HIV/AIDS come from? How is HIV spread? What are the symptoms and treatments?
Researchers found that a similar disease occurs in chimpanzees, other monkeys, domestic livestock, and lions. Most scientists accept the theory that hunters living in Cameroon, West Africa, were the first to contract this HIV-like illness from improperly cooked bush meat, such as chimpanzees. And it’s believed that it took two hundred to four hundred years to morph into what we now call HIV, moving into Europe in 1939 with returning settlers. Several cases of HIV have been documented from the mid-1960s. The disease reached epidemic proportions in the 1980s.
Unlike some highly infectious viruses, HIV is not simple to catch. It doesn’t spread through the air, water, or insect bites. HIV can’t survive on household surfaces. In a health-care setting, spilled blood is cleaned up with a disinfectant as a precaution. HIV is spread from an infected or infectious person’s blood and reproductive fluids—semen, breast milk, and vaginal secretions. If a healthy person comes in direct contact with infected reproductive fluids or blood by mouth, needle, or sexual activity, or through an open cut, HIV can develop.
By 1985, a blood test was invented to identify HIV-positive people. Since then, blood and organ donors are screened, eliminating these sources of infection. But HIV can still pass through the placenta of an infected mother into the bloodstream of a fetus.
By early 2010, 33.3 million people worldwide were HIV positive—that’s about the same as the population of Canada. Twenty-five million died of AIDS between 1981 and 2011.
Symptoms of HIV include rapid weight loss, ongoing diarrhea, night sweats, swollen glands, memory loss, depression, and skin blotches. Without treatment, full-blown AIDS takes hold and death by infection or cancer will follow within about ten years.
Billions of dollars have been spent searching for an HIV/AIDS vaccine, but because the HIV virus changes very rapidly, there is no vaccine yet. Combinations of antiviral drugs, sometimes called cocktails, keep the virus from entering the AIDS phase and allow HIV patients to lead a “normal” life. The price tag is huge, so millions of HIV patients cannot afford treatment. Scientists are urgently searching for better, cheaper drugs and a vaccine, hoping to neutralize the virus before it changes and outsmarts the current treatment.
Without a cure or a vaccine, prevention is still the best solution. Education about the importance of clean needles and safe sex using condoms has lowered infection rates. But HIV remains a huge menace in Africa, home to 68 percent of all people living with the disease. The United Nations estimates that 14.8 million children in sub-Saharan Africa are orphans of parents who died of AIDS, living on their own or being raised by their grandparents, older siblings, or caregivers in orphanages.
AIDS has drastically changed the health of whole populations. In Botswana, for example, the average lifespan was over sixty years in the late 1960s but has now dropped to mid-forties. The disease kills the working segment of the population—mothers and fathers who put food on the table; who earn money for clothes, education, and health care; and who bind families together with love and caring. In the worst-hit parts of Africa, it is common for people to deny illness and to avoid testing because an HIV-positive test result translates into dying alone, cast out from family and community.
Although scientists predict we will see more new infections in the future—maybe worse than AIDS—there is some hope. Education and prevention programs, such as the Global Health Program, sponsored by the Bill and Melinda Gates Foundation, have increased the number of sub-Saharan Africans who get tested for HIV and receive free drugs. The goal is to stop the transmission of the virus and to enter a time when all communities test negatively—just as the three Africans did in the village on the shores of Lake Victoria, Tanzania, in 2004.