References
Adler
D, Price
JH. Relation of agoraphobics’ health locus of control orientation to severity of agoraphobia. Psychological Reports. 1985;56:619
–
625.
Anderson
CA, Arnoult
LH. Attributional style and everyday problems in living: Depression, loneliness, and shyness. Social Cognition. 1985;3:16
–
35.
Antonuccio
DO, Thomas
M, Danton
WG. A cost-effectiveness analysis of cognitive behavior therapy and fluoxetine (Prozac) in the treatment of depression. Behavior Therapy. 1997;28:187
–
210.
Bandura
A. Self-efficacy: toward a unifying theory of behavioral change. Psychological Review. 1977;84
(2)
:
191
–
215.
Barlow
DH, Gorman
JM, Shear
MK, Woods
SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. Journal of the American Medical Association. 2000;283:2529
–
2536.
Basoglu
M, Marks
IM, Kilic
C, Brewin
CR, Swinson
RP. Alprazolam and exposure for panic disorder with agoraphobia attribution of improvement to medication predicts subsequent relapse. British Journal of Psychiatry. 1994;164:652
–
659.
Biondi
M, Picardi
A. Attribution of improvement to medication and increased risk of relapse of panic disorder with agoraphobia. Psychotherapy and Psychosomatics. 2003;72:110
–
111.
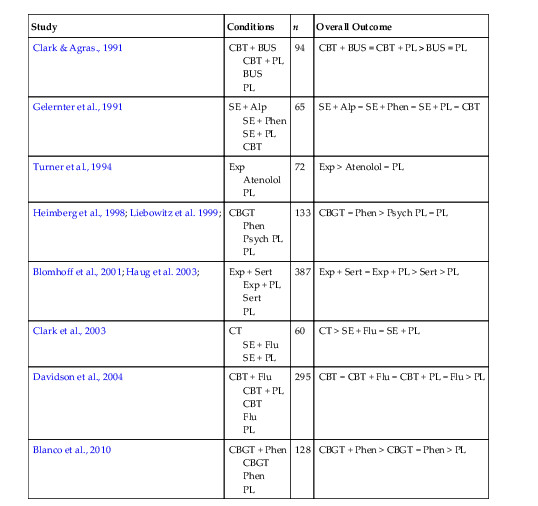
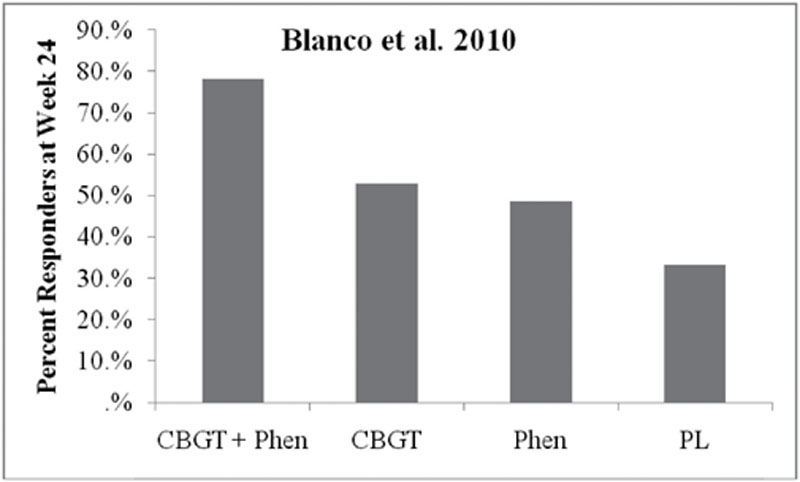
Blanco
C, Heimberg
RG, Schneier
FR, Fresco
DM, Chen
H, Turk
CL
,
et al.
A placebo-controlled trial of phenelzine, cognitive behavioral group therapy, and their combination for social anxiety disorder. Archives of General Psychiatry. 2010;67
(3)
:
286
–
295.
Blomhoff
S, Haug
TT, Hellström
K, Holme
I, Humble
M, Madsbu
HP, Wold
JE. Randomised controlled general practice trial of sertraline, exposure therapy and combined treatment in generalized social phobia. British Journal of Psychiatry. 2001;179:23
–
30.
Bouton
ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976
–
986.
Broadbeck
C, Michelson
L. Problem-solving skills and attributional styles of agoraphobics. [10.1007/BF01183861]. Cognitive Therapy and Research. 1987;11
(5)
:
593
–
610.
Cain
CK, Blouin
AM, Barad
M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learning & Memory. 2004;11:179
–
187.
Clark
DB, Agras
WS. The assessment and treatment of performance anxiety in musicians. American Journal of Psychiatry. 1991;148
(5)
:
598
–
605.
Clark
DM, Ehlers
A, McManus
F, Hackmann
A, Fennell
M, Campbell
H
,
et al.
Cognitive therapy versus fluoxetine in generalized social phobia: A randomized placebo- controlled trial. Journal of Consulting and Clinical Psychology. 2003;71
(6)
:
1058
–
1067.
Cloitre
M, Heimberg
RG, Liebowitz
MR, Gitow
A. Perceptions of control in panic disorder and social phobia. Cognitive Therapy and Research. 1992;16:569
–
577.
Cowley
DS, Ha
EH, Roy-Byrne
PP. Determinants of pharmacologic treatment failure in panic disorder. Journal of Clinical Psychiatry. 1997;58
(12)
:
555
–
561.
Craske
MG, Golinelli
D, Stein
MB, Roy-Byrne
P, Bystritsky
A, Sherbourne
C. Does the addition of cognitive behavioral therapy improve panic disorder treatment outcome relative to medication alone in the primary-care setting? Psychological Medicine. 2005;35:1645
–
1654.
D’Souza
DC, Gil
R, Cassello
K, Morrissey
K, Abi-Saab
D, White
J, Sturwold
R, Bennett
A, Karper
LP, Zuzarte
E, Charney
DS, Krystal
JH. IV glycine and oral D-cycloserine effects on plasma and CSF amino acids in healthy humans. Biological Psychiatry. 2000;47:450
–
462.
Davidson
JRT, Foa
EB, Huppert
JD, Keefe
FJ, Franklin
ME, Compton
JS, Zhao
N, Connor
KM, Lynch
TR, Gadde
KM. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Archives of General Psychiatry. 2004;61:1005
–
1013.
Davis
M. Role of NMDA receptors and MAP kinase in the amygdala in extinction of fear: Clinical implications for exposure therapy. European Journal of Neuroscience. 2002;16:395
–
398.
Davis
M, Falls
WA, Gewirtz
J. Neural systems involved in fear inhibition: Extinction and conditioned inhibition.
In:
Myslobodsky
M
,
Weiner
I
,
eds.
Contemporary issues in modeling psychopathology
.
Boston: Kluwer Academic Publishers; 2000:113
–
142.
Davis
M, Myers
KM. The role of glutamate and gamma-aminobutyric acid in fear extinction: Clinical implications for exposure therapy. Biological Psychiatry. 2002;52:998
–
1007.
Deacon
BJ, Abramowitz
JS. Patients’ perceptions of pharmacological and cognitive-behavioral treatments for anxiety disorders. Behavior Therapy. 2005;36:139
–
145.
Emmelkamp
PM, Cohen-Kettenis
PT. Relationship of locus of control to phobic anxiety and depression. Psychological Reports. 1975;36:390.
Fang, A., Hoge, E.A., Heinrichs, M., & Hofmann, S.G. (in press) Attachment style moderaters the effects of oxytocin on social behaviors and cognitions during social rejection: Applying an RdoC framework to social anxiety. Clinical Psychological Science.
Fedoroff
IC, Taylor
S. Psychological and pharmacological treatments of social phobia: a meta-analysis. Journal of Clinical Psychopharmacology. 2001;21
(3)
:
311
–
324.
Foa
EB, Kozak
MJ. Emotional processing of fear: Exposure to corrective information. Psychological Bulletin. 1986;99:20
–
35.
Foa
EB, Liebowitz
MR, Kozak
MJ, Davies
S, Campeas
R, Franklin
ME, Huppert
JD, Kjernisted
K, Rowan
V, Schmidt
AB, Simpson
HB, Tu
X. Randomized, Placebo-Controlled Trial of Exposure and Ritual Prevention, Clomipramine, and Their Combination in the Treatment of Obsessive-Compulsive Disorder. The American Journal of Psychiatry. 2005;162
(1)
:
151
–
161.
Gelernter
CS, Uhde
TW, Cimbolic
P, Arnkoff
DB, Vittone
BJ, Tancer
ME
,
et al.
Cognitive-behavioral and pharmacological treatments of social phobia: A controlled study. Archives of General Psychiatry. 1991;48
(10)
:
938
–
945.
Gorman
JM, Kent
JM, Sullivan
GM, Coplan
JD. Neuroanatomical hypothesis of panic disorder, revised. American Journal of Psychiatry. 2000;157
(4)
:
493
–
505.
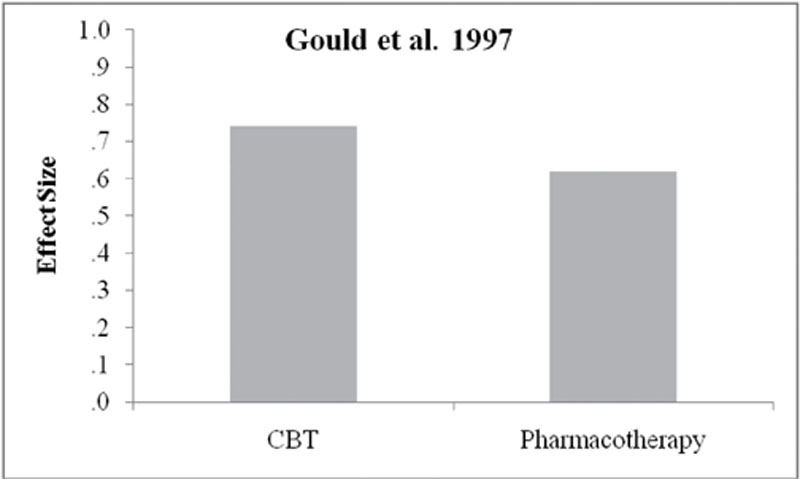
Gould
RA, Buckminster
S, Pollack
MH, Otto
MW, Yap
L. Cognitive-behavioral and pharmacological treatment for social phobia: A meta-analysis. Clinical Psychology: Science and Practice. 1997;4:291
–
306.
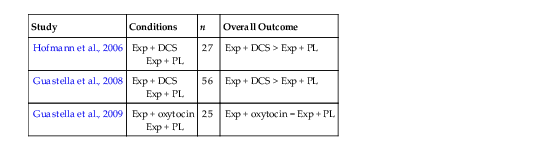
Guastella
AJ, Howard
AL, Dadds
MR, Mitchell
P, Carson
DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34
(6)
:
917
–
923.
Guastella
AJ, Richardson
R, Lovibond
PF, Rapee
RM, Gaston
JE, Mitchell
P
,
et al.
A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biological Psychiatry. 2008;63
(6)
:
544
–
549.
Haug
TT, Blomhoff
S, Hellström
K, Holme
I, Humble
M, Madsbu
HP, Wold
JE. Exposure therapy and sertraline in social phobia: One-year follow-up of a randomised controlled trial. British Journal of Psychiatry. 2003;182:312
–
318.
Hedman
E, Furmark
T, Carlbring
P, Ljótsson
B, Rück
C, Lindefors
N, Andersson
G. A 5-Year follow-up of internet-based cognitive behavior therapy for social anxiety disorder. Journal Of Medical Internet Research. 2011;13
(2)
:
e39
..
Heimberg
R, Liebowitz
MR, Hope
DA, Schneier
FR, Holt
CS, Welkowitz
LA
,
et al.
Cognitive behavioral group therapy vs. phenelzine therapy for social phobia: 12-week outcome. Archives of General Psychiatry. 1998;55
(12)
:
1133
–
1141.
Heimberg
RG, Salzman
DG, Holt
CS, Blendell
KA. Cognitive-behavioral group treatment for social phobia: Effectiveness at five-year follow-up. Cognitive Therapy and Research. 1993;17:325
–
339.
Heldt
E, Manfro
GG, Kipper
L, Blaya
C, Isolan
L, Otto
MW. One-year follow-up of pharmacotherapy-resistant patients with panic disorder treated with cognitive-behavior therapy: Outcome and predictors of remission. Behaviour Research and Therapy. 2006;44:657
–
665.
Heresco-Levy
U, Kremer
I, Javitt
DC, Goichman
R, Reshef
A, Blanaru
M, Cohen
T. Pilot-controlled trial of D-cycloserine for the treatment of post-traumatic stress disorder. International Journal of Neuropsychopharmacology. 2002;5:301
–
307.
Herry
C, Garcia
R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. Journal of Neuroscience. 2002;22:577
–
583.
Heuzenroeder
L, Donnelly
M, Haby
MM, Mihalopoulos
C, Rossell
R, Carter
R, Andrews
G, Vos
T. Cost-effectiveness of psychological and pharmacological interventions for generalized anxiety disorder and panic disorder. Australian and New Zealand Journal of Psychiatry. 2004;38:602
–
612.
Hoffart
A, Martinsen
EW. Agoraphobia, depression, mental health locus of control, and attributional styles. Cognitive Therapy and Research. 1990;14:343
–
351.
Hofmann, S.G. (in press). D-cycloserine for treating anxiety disorders: Making good exposures better and bad exposures worse. Depression and Anxiety.
Hofmann
SG, Barlow
DH, Papp
LA, Detweiler
MF, Ray
SE, Shear
MK, Woods
SW, Gorman
JM. Pretreatment attrition in a comparative treatment outcome study on panic disorder. American Journal of Psychiatry. 1998;155:43
–
47.
Hofmann
SG, Meuret
AE, Smits
JAJ, Simon
NM, Pollack
MH, Eisenmenger
K, Shiekh
M, Otto
MW. Augmentation of exposure therapy with d-cycloserine for social anxiety disorder. Archives of General Psychiatry. 2006;63:298
–
304.
Hofmann
SG, Sawyer
AT, Korte
KJ, Smits
JAJ. Is it beneficial to add pharmacotherapy to cognitive-behavioral therapy when treating anxiety disorders? A meta-analytic review. International Journal of Cognitive Therapy. 2009;2:160
–
175.
Hofmann
SG, Smits
JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. Journal of Clinical Psychiatry. 2008;69:621
–
632.
Hofmann
S, Smits
J, Rosenfield
D, Simon
N, Otto
M, Meuret
A, Pollack
M. D-Cycloserine as an augmentation strategy with cognitive-behavioral therapy for social anxiety disorder. The American Journal Of Psychiatry. 2013;170
(7)
:
751
–
758
..
Kamphuis
JH, Telch
MJ. Effect of distraction and guided threat reappraisal on fear reduction during exposure-based treatments for specific fears. Behaviour Research and Therapy. 2000;38
(12)
:
1163
–
1181.
Kampman
M, Keijsers
GP, Hoogduin
CA, Hendriks
GJ. A randomized, double-blind, placebo-controlled study of the effects of adjunctive paroxetine in panic disorder patients unsuccessfully treated with cognitive-behavioral therapy alone. Journal of Clinical Psychiatry. 2002;63:772
–
777.
Kampman
M, Keijsers
GPJ, Hoogdiun
CAL, Verbraak
MJPM. Addition of cognitive-behaviour therapy for obsessive-compulsive disorder patients non-responding to fluoxetine. Acta Psychiatrica Scandinavica. 2002;106:314
–
319.
Ledgerwood
L, Richardson
R, Cranney
J. D-cycloserine facilitates extinction of learned fear: effects of reacquisition and generalized extinction. Biological Psychiatry. 2005;57:841
–
847.
Liebowitz
MR, Heimberg
RG, Schneier
F, Hope
DA, Davies
S, Holt
CS, Goetz
D, Juster
HR, Lisn
SH, Bruch
MA, Marshall
RD, Klein
DF. Cognitive-behavioral group therapy versus phenelzine in social phobia: long term outcome. Depression and Anxiety. 1999;10:89
–
98.
Lydiard
RB, Brawman-Mintzer
O, Ballenger
JC. Recent developments in the psychopharmacology of anxiety disorders. Journal of Consulting and Clinical Psychology. 1996;64:660
–
668.
Marks
IM, Lelliott
P, Basoglu
M, Noshirvani
H, Monteiro
W, Cohen
D, Kasvikis
Y. Clomipramine, self-exposure and therapist-aided exposure for obsessive-compulsive rituals. British Journal of Psychiatry. 1988;152:522
–
534.
Marks
IM, Swinson
RP, Basaglu
M, Kuch
K, Nasirvani
H, O’Sullivan
G, Lelliott
PT, Kirby
M, McNamee
G, Sengun
S
,
et al.
Alprazolam and exposure alone and combined in panic disorder with agoraphobia: A controlled study in London and Toronto. British Journal of Psychiatry. 1993;162:776
–
787.
Mavissakalian
M, Michelson
L. Agoraphobia: Relative and combined effectiveness of therapist-assisted in vivo exposure and imipramine. Journal of Clinical Psychiatry. 1986;47
(3)
:
117
–
122.
Mavissakalian
M, Perel
JM. Clinical experiments in maintenance and discontinuation of imipramine therapy in panic disorder with agoraphobia. Archives of General Psychiatry. 1992;49:318
–
323.
Mavissikalian
M, Perel
JM. Clinical experience in maintenance and discontinuation of imipramine therapy in panic disorder with agoraphobia. Archives General Psychiatry. 1993;49:318
–
323.
Menezes
G, Coutinho
E, Fontenelle
L, Vigne
P, Figueira
I, Versiani
M. Second-generation antidepressants in social anxiety disorder: meta-analysis of controlled clinical trials. Psychopharmacology. 2011;215
(1)
:
1
–
11
..
McHugh
RK, Otto
MW, Barlow
DH, Gorman
JM, Shear
MK, Woods
SW. Cost-efficacy of individual and combined treatments of panic disorder. Journal of Clinical Psychiatry. 2007;68:1038
–
1044.
Milad
MR, Quirk
GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70
–
74.
Morgan
MA, Romanski
LM, LeDoux
JE. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neuroscience Letters. 1993;163:109
–
113.
Mystkowski
JL, Mineka
S, Vernon
LL, Zinbarg
RE. Changes in caffeine states enhance return of fear in spider phobia. Journal of Consulting and Clinical Psychology. 2003;71:243
–
250.
Noyes
R, Garvey
MJ, Cook
BL, Samuelson
L. Problems with tricyclic antidepressant use in patients with panic disorder or agoraphobia: results of a naturalistic follow-up study. Journal of Clinical Psychiatry. 1989;50:163
–
169.
Noyes
R, Garvey
MJ, Cook
B, Suelzer
M. Controlled discontinuation of benzodiazepine treatment for patients with panic disorder. American Journal of Psychiatry. 1991;148:517
–
523.
Otto
MW, Hinton
D, Korbly
NB, Chea
A, Phalnarith
B, Gershuny
BS, Pollack
MH. Treatment of pharmacotherapy-refractory posttraumatic stress disorder among Cambodian refugees: A pilot study of combination treatment with cognitive-behavior therapy vs. sertraline alone. Behaviour Research and Therapy. 2003;41:1271
–
1276.
Otto
MW, Pollack
MH, Maki
KM. Empirically-supported treatment for panic disorder: Costs, benefits, and stepped care. Journal of Consulting and Clinical Psychology. 2000;68:556
–
563.
Otto
MW, Pollack
MH, Penava
SJ, Zucker
BG. Cognitive-behavior therapy for patients failing to respond to pharmacotherapy for panic disorder: A clinical case series. Behaviour Research and Therapy. 1999;37:763
–
770.
Otto
MW, Pollack
MH, Sabatino
SA. Maintenance of remission following cognitive-behavior therapy for panic disorder: Possible deleterious effects of concurrent medication treatment. Behavior Therapy. 1996;27:473
–
482.
Otto
MW, Pollack
MH, Sachs
GS, Reiter
SR, Meltzer-Brody
S, Rosenbaum
JF. Discontinuation of benzodiazepine treatment: Efficacy of cognitive-behavioral therapy for patients with panic disorder. American Journal of Psychiatry. 1993;150
(10)
:
1485
–
1490.
Otto
MW, Safren
SA, Nicolaou
DC, Pollack
MH. Considering mechanisms of action in the treatment of social anxiety disorder.
In:
Pollack
MH
,
Simon
NM
,
Otto
MW
,
eds.
Social Phobia: Presentation, course, and treatment
.
New York: Castle Connolly Graduate Medical Publishing; 2003.
Otto
MW, Smits
JAJ, Reese
HE. Cognitive-behavioral therapy for the treatment of anxiety disorders. Journal of Clinical Psychiatry. 2004;65
(Suppl 5)
:
34
–
41.
Parnas
AS, Weber
M, Richardson
R. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiology of Learning and Memory. 2005;83
(3)
:
224
–
231.
Pollack
MH, Otto
MW, Kaspi
SP, Hammerness
PG, Rosenbaum
JF. Cognitive-behavior therapy for treatment-refractory panic disorder. Journal of Clinical Psychiatry. 1994;55:200
–
205.
Pollack
MH, Smoller
JW. Pharmacologic approaches to treatment resistant panic disorder.
In:
Pollack
MH
,
Otto
MW
,
eds.
Challenges in clinical practice: Pharmacological and psychosocial strategies
.
New York: Guilford Press; 1996:89
–
112.
Powers
MB, Sigmarsson
SR, Emmelkamp
PMG. A meta-analytic review of social phobia treatments. International Journal of Cognitive Therapy. 2008;1:94
–
113.
Powers
MB, Smits
JAJ, Leyro
TM, Otto
M. Translational research perspectives on maximizing the effectiveness of exposure therapy.
In:
Richard
DCS
,
Lauterbach
D
,
eds.
Comprehensive handbook of the exposure therapies
.
New York: Academic Press; 2006.
Powers
MB, Smits
JAJ, Otto
MW, Sanders
C, Emmelkamp
PMG. Facilitation of fear extinction in phobic participants with a novel cognitive enhancer: A randomized placebo controlled trial of yohimbine augmentation. Journal of Anxiety Disorders. 2009;23
(3)
:
350
–
356.
Powers
MB, Smits
JA, Telch
MJ. Disentangling the effects of safety-behavior utilization and safety-behavior availability during exposure-based treatment: a placebo-controlled trial. Journal of Consulting and Clinical Psychology. 2004;72
(3)
:
448
–
454.
Powers
MB, Smits
JA, Whitley
D, Bystritsky
A, Telch
MJ. The effect of attributional processes concerning medication taking on return of fear. Journal of Consulting & Clinical Psychology. 2008;76:478
–
490.
Quartermain
D, Mower
J, Rafferty
MF, Herting
RL, Lanthorn
TH. Acute but not chronic activation of the NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. European Journal of Pharmacology. 1994;157:7
–
12.
Quirk
GJ, Russo
GK, Barron
JL, Lebron
K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. Journal of Neuroscience. 2000;20:6225
–
6231.
Ressler
KJ, Rothbaum
BO, Tannenbaum
L, Anderson
P, Graap
K, Zimand
E, Hodges
L, Davis
M. Cognitive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobics to facilitate extinction of fear. Archives of General Psychiatry. 2004;61
(11)
:
1136
–
1144.
Richardson
R, Ledgerwood
L, Cranney
J. Facilitation of fear extinction by D-cycloserine: theoretical and clinical implications. Learning and Memory. 2004;11:510
–
516.
Rodriguez
BI, Craske
MG. The effects of distraction during exposure to phobic stimuli. Behaviour Research and Therapy. 1993;31:549
–
558.
Sirey
JA, Meyers
BS, Bruce
ML, Alexopoulos
GS, Perlick
DA, Raue
P. Predictors of antidepressant prescription and early use among depressed outpatients. American Journal of Psychiatry. 1999;156
(5)
:
690
–
696.
Sloan
T, Telch
MJ. The effects of safety-seeking behavior and guided threat reappraisal on fear reduction during exposure: an experimental investigation. Behaviour Research and Therapy. 2002;40:235
–
251.
Smits
JA, Rosebfield
D, Davis
ML, Julian
K, Handelsman
PR, Otto
MW, Tuerk
P, Shiekh
M, Rosenfield
B, Hofmann
SG, Powers
MB. Yohimbine Enhancement of Exposure Therapy for Social Anxiety Disorder: A randomized Controlled Trial. Society of Biological Psychiatry. 2013
.
.
Smits
JA, Rosenfield
D, Otto
MW, Marques
L, Davis
ML, Meuret
AE, Hofmann
SG. d-cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. Journal Of Psychiatric Research. 2013;47
(10)
:
1455
–
1461
..
Smoller
JW, Pollack
MH. Pharmacologic approaches to treatment resistant social phobia and generalized anxiety disorder.
In:
Pollack
MH
,
Otto
MW
,
eds.
Challenges in Clinical Practice: Pharmacological and Psychosocial Strategies
.
New York: Guilford Press; 1996:141
–
170.
Stein
DJ, Versiani
M, Hair
T, Kumar
R. Efficacy of paroxetine for relapse prevention in social anxiety disorder. Archives of General Psychiatry. 2002;59:1111
–
1118.
Sutherland
SM, Davidson
JRT. β-Blockers and benzodiazepines in pharmacotherapy.
In:
Stein
MB
,
ed.
Social phobia: Clinical and research perspectives
.
Washington, DC: American Psychiatric Press; 1995:323
–
326.
Telch
MJ, Agras
WS, Taylor
CB, Roth
WT, Gallen
C. Combined pharmacological and behavioral treatment for agoraphobia. Behaviour Research and Therapy. 1985;23:325
–
335.
Titov
N, Andrews
G, Johnston
L, Schwencke
G, Choi
I. Shyness programme: longer term benefits, cost-effectiveness, and acceptability. Australian & New Zealand Journal Of Psychiatry. 2009;43
(1)
:
36
–
44
..
Turner
SM, Beidel
DC, Jacob
RG. Social phobia: A comparison of behavior therapy and atenolol. Journal of Consulting and Clinical Psychology. 1994;62
(2)
:
350
–
358.
van Berckel
BN, Lipsch
C, Gispen-de Wied
C, Wynne
HJ, Blankenstein
MA, van Ree
JM, Kahn
RS. The partial NMDA agonist D-cycloserine stimulates LH secretion in healthy volunteers. Psychopharmacology (Berl). 1998;138:190
–
197.
Walker
JR, Van Ameringen
MA, Swinson
R, Bowen
RC, Chokka
PR, Goldner
E
,
et al.
Prevention of relapse in generalized social phobia: Results of a 24-week study in responders to 20 weeks of sertraline treatment. Journal of Clinical Psychopharmacology. 2000;20:636
–
644.
Weilburg
JB, O’Leary
KM, Meigs
JB, Hennen
J, Stafford
RS. Evaluation of the adequacy of outpatient antidepressant treatment. Psychiatric Services. 2003;54
(9)
:
1233
–
1239.
Wersebe
H, Sijbrandij
M, Cuijpers
P. Psychological group-treatments for social anxiety disorder: A meta-analysis. PLoS ONE. 2013;11:1
–
4.
Whittal
ML, Otto
MW, Hong
JJ. Cognitive-behavior therapy for discontinuation of SSRI treatment of panic disorder: a case series. Behavior Research and Therapy. 2001;8:939
–
945.