TECHNOSPHERE IMPACTS ON THE GLOBAL BIOGEOCHEMICAL CYCLES
The human expansion and domination of the Earth system over the past 10,000 years, albeit fleetingly recent in the 4.6 billion year context of Earth history, represents a major transition in the nature of life on Earth, comparable energetically to the colonization of land by plants.
—Y. Malhi (2014)
THE GLOBAL BIOGEOCHEMICAL CYCLES
Solar energy is used by the biosphere to generate and maintain order in the form of living organisms, ecosystems, and the biosphere itself. As noted in chapter 2, Earth is largely a closed system with respect to mass; thus, nutrients must be cycled to maintain biosphere productivity. Scientists speak of biogeochemical cycles because the chemical elements that make up living biomass are repeatedly transformed, moving in different chemical forms through phases in the geosphere and biosphere, as well as the atmosphere and hydrosphere in some cases.
The global biogeochemical cycles are each quite complex and involve myriad chemical compounds and chemical reactions. Many of those reactions are driven by the metabolism of a wide array of microbes and higher life forms, while others are purely inorganic. The biogeochemical cycles of some elements, such as carbon and nitrogen, are closely linked because both are assimilated by growing organisms. Despite the complexity of the global biogeochemical cycles, Earth system science has made considerable progress in characterizing the relevant chemical reactions, quantifying their rates at the local scale, and extrapolating (scaling up) the estimates for stocks (the amount present) and fluxes (transfers from one pool to another) to the global scale. This information is often summarized in box and arrow diagrams (e.g., figure 4.1).
The human enterprise (i.e., the technosphere) has added a new overlay of elemental transformations that tends to distort and to speed up the preexisting biogeochemical cycles (Zalasiewicz et al., 2016). Because the human overlay of biogeochemical transformations is a product of cultural evolution (box 4.1) rather than biological evolution, it can introduce into managed and unmanaged ecosystems highly artificial concentrations and forms of chemical compounds, and very rapid rates of chemical reactions. Ecosystem-regulating mechanisms may thus become damaged, with consequent loss of system integrity. There is ample evidence for human-induced ecosystem dysfunction at the local scale (e.g., eutrophication of lakes), but we must now diagnose and treat possible global-scale disruptions.

FIGURE 4.1
The fast carbon cycle on land. Stocks (“pools”) that are “sources” supply carbon to another pool. “Sinks” receive carbon from another pool.
BOX 4.1
Cultural Niche Construction
How has one species, Homo sapiens, come to dominate the biosphere? The history of human influence on the biosphere and climate is well documented (e.g., Ruddiman, 2005), but the underlying mechanism is less clear. This question is worth asking since providing an answer in scientific terms could help us design a more sustainable relationship of humanity to the rest of the Earth system. Two key aspects of the answer are niche construction and cultural evolution (Ellis, 2015).
Niche construction was introduced in chapter 3 in the context of theoretical efforts to integrate ecosystem ecology and evolutionary biology. The ecosystem engineering of beaver is the paradigmatic example of niche construction. In that case, the way the beaver changes its environment affects the selection regime for itself and many other species. A young beaver is born into a constructed environment (its ecological inheritance).
Homo sapiens added a new wrinkle to reliance on niche construction by basing it on cultural evolution (learned behavior) rather than genetic evolution (mostly programmed behavior). The power of cultural evolution (i.e., based on the transmission of information across generations) is that it can proceed much more rapidly than genetic evolution. Like biological evolution, the products of cultural evolution are preserved across generations and build on each other (i.e., a “ratchet effect” prevails). With humans, the constructed niche (in terms of material culture such as buildings and ecosystem modifications such as agriculture) began to be specified by the products of cultural evolution. Ellis (2015, p. 293) refers to cultural niche construction as “alteration of ecological patterns and processes by organisms through socially learned behaviors that produce heritable advantages and/or disadvantages to individuals or populations.”
With humans, inheritance has thus come to include four components: genetic inheritance (e.g., big brains), cultural inheritance (e.g., information transmitted mostly by language), material inheritance (e.g., walled cities), and ecological inheritance (e.g., agricultural ecosystems). Thus, a baby is born into—and benefits from—a highly constructed environment, and is in a position to rapidly learn a tremendous amount about the world from the members of its community.
This evolutionary strategy relies on language ability, and no other species has specialized in quite this way. A key problem now is that the ecological inheritance we are passing on has come to include characteristics of the global environment, and the traits by which our impact on the global environment are manifest may no longer be adaptive.
The essential premise of Vernadsky’s noösphere concept (introduced in chapter 1) is that humanity can learn enough about the global biogeochemical cycles to successfully manage the impact of the technosphere on them. Supporting an environment favorable to long-term human habitation of the planet depends on fulfilling Vernadsky’s vision. Indeed, we must become a component in a teleological feedback to Earth system change (Lenton, 2016).
The text in this chapter is a synoptic survey, for the nonchemist, of human impacts on the biogeochemical cycles. It is intended primarily to give an indication of the degree to which the technosphere has altered their background operation. Smil (2002) and Schlesinger and Bernhart (2013) go one step deeper in terms of explaining the chemistry and biology of specific elemental cycles and their global budgets. The text by Lenton and Watson (2011) discusses in greater chemical detail some of the interesting recent controversies in the global biogeochemistry literature.
THE EMERGENCE OF THE TECHNOSPHERE
The nutrient cycling capabilities of the biosphere have evolved over its approximately 3.7-billion-year lifetime. Much of the biochemical machinery that underlies the metabolism of microbes, plants, and animals is similar across life forms; e.g., the reliance on DNA for storing information, and on the set of 20 or so critical amino acids for building enzymatic compounds. However, biological evolution has vastly altered the structural forms and functional capabilities of distinct species.
One noted anatomical trend among animal species is toward ever more elaborate nervous systems and brains. Homo sapiens is clearly the leading edge of that trend. Nervous systems and brains offer many advantages in the Darwinian struggle for existence, and Professor of Evolutionary Paleobiology Simon Conway Morris nicely lays out the evolutionary sequence leading to the large brains in Homo sapiens (Conway Morris, 2015). Those brains are apparently required to support consciousness, and consciousness is required to generate a technosphere.
The emergence of “thinking matter” from the biosphere is as profound a change in the Earth system as was the emergence of living matter from the geosphere. We earlier noted the apparent order-friendly and life-friendly quality of the universe; from the astrobiology community, we also have the suggestion that we live in a consciousness-friendly universe (Martinez, 2014). Again, although an unscientific proposition (i.e., untestable), the implication is that we are in some sense “at home in the universe” (Kauffman, 1995) and conceivably have the potential to build a sustainable, high-technology planetary civilization.
It is worth briefly tackling the subject of consciousness here because this form of brain functioning is so important to understanding both the history of human interaction with the biosphere and our prospects (Vandenburg, 1985). Consciousness is notoriously tricky to define, and to keep our level of abstraction manageable, let’s mostly equate it with its highest form, i.e., self-awareness. One aspect of consciousness is the ability to know oneself as having existed in the past, as existing in the present, and as likely to exist in the future. Consciousness certainly involves thinking (cognition; i.e., the mental capacity to juggle words, concepts, and images). Consciousness implies the capacity for metacognition (thinking about or awareness of one’s own thoughts). A related ability is the capacity for a “theory of mind”: the ability to view another person as an intentional being and to imagine what he or she is thinking.
With consciousness, behavior can increasingly be shaped by social learning rather than genetic programming (which accounts for much of animal behavior). The earliest forms of thought, leading to a capacity for language, were based on imagination and mythology. Primitive language, along with a limited ability to associate cause and effect, was enough to transition from the hunter-gatherer way of life to one supported by agriculture. Gradually, thinking became more self-reflective and less referenced to anthropomorphic myths. Since about 1500 CE, we have gained the capacity for scientific thought, a key feature of which is the search for natural laws by way of observations and experimentation. The application of scientific thought transformed the relationship of humanity to the geosphere and biosphere. It allowed us to build the technosphere, a web of artifacts and machine-driven processes that has proliferated wildly over the surface of the planet in the past 100 years. As Vernadsky pointed out, we have become a geological force.

FIGURE 4.2
Earth system indicator trends (1750–2010) associated with the Great Acceleration. Adapted from Steffen et al. (2015a).
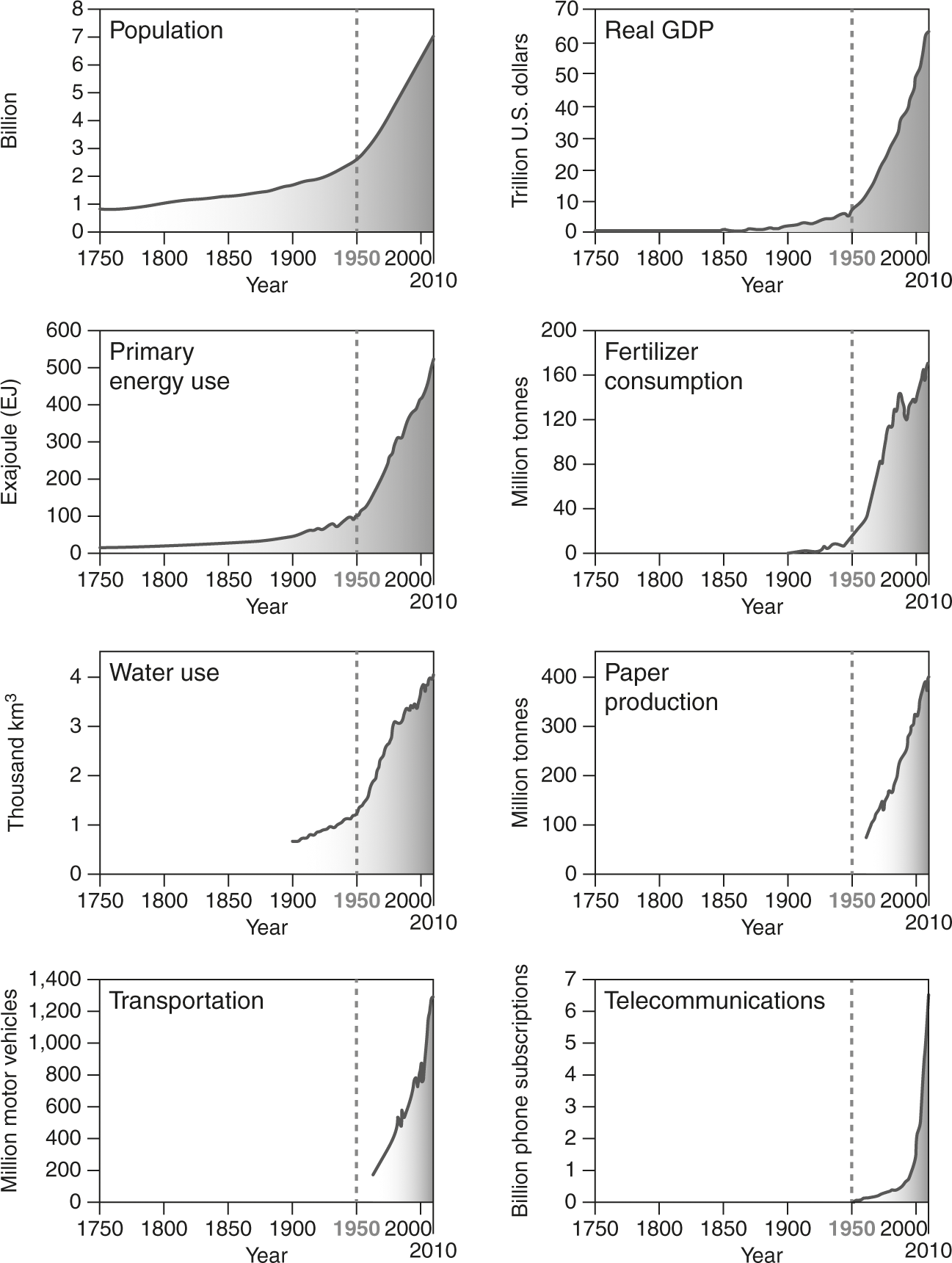
FIGURE 4.3
Socioeconomic indicator trends (1750–2010) associated with the Great Acceleration. Adapted from Steffen, Broadgate, Deutsch, Gaffney, and Ludwig (2015a).
The emergence and ascendency of the technosphere is covered by the Building the Technosphere and Great Acceleration phases of the Anthropocene narrative (introduced in chapter 1). Building the Technosphere includes both the technological aspects of the Industrial Revolution and the socioeconomic changes associated with the rise of market capitalism. The Great Acceleration refers to the period after 1950 when multiple indicators of technosphere expansion and economic development entered a phase of exponential growth (figures 4.2 and 4.3; Steffen, Broadgate, Deutsch, Gaffney, and Ludwig, 2015a). Efforts to explain the extraordinary success of the human species in the context of the Extended Evolutionary Synthesis (introduced in chapter 3) are ongoing (box 4.1).
CARBON
In chapter 2 we discussed the slow geochemical cycling of carbon (C) between a small pool in a gaseous form in the atmosphere, a large pool as a soluble form in the ocean, and the large below-ground pools in coal and oil formations, as well as in carbonate rocks. The carbon atom cycling time of this slow, mostly geochemical, pathway is millions of years. With the development of the biosphere, an alternative and much faster pathway of carbon cycling—among producers, consumers, decomposers, and the atmosphere—was established (figure 4.1). On land, carbon is taken up from the atmosphere by plants, stored in live biomass, transferred to a dead biomass pool at the time of plant death, digested by consumers or decomposer organisms, and eventually returned to the atmosphere. There is a parallel cycle in the ocean, with uptake dominated by photosynthetic plankton. The cycling time for this faster loop is years to centuries.
The technosphere has significantly disrupted both the slow and fast loops of the carbon cycle. The background global emissions of carbon from volcanism are on the order of 0.2 PgC (petagrams of carbon) per year (1 Pg = 1015 g, or 1,000,000,000,000,000 grams). Currently, the burning of fossil fuel plus industrial emissions (e.g., cement manufacture) introduces close to 9 PgC to the atmosphere each year (Le Quere et al., 2016); that is nearly 50 times the background geosphere source. The background carbon uptake (sink) associated with silicate rock weathering and the biological pump in the ocean (described in chapter 2) is presumably about the same magnitude as the tectonic source (0.2 PgC per year) because the atmospheric carbon dioxide (CO2) concentration was mostly stable prior to the recent surge in emissions. The rate of silicate rock weathering is probably not increasing significantly in response to recent climate warming (Colbourn, Ridgwell, and Lenton, 2015).
With regard to the fast carbon cycle pathway, the technosphere is doing several things. First, we (its agents) are transferring large quantities of carbon directly from the live and dead biomass pools to the atmosphere in the process of deforestation. This transfer is on the order of 1.0 PgC per year in recent years (Le Quere et al., 2016). Areas of forest loss are mostly in the tropical zone, e.g., conversion to soybean fields in Brazil and to palm oil plantations in Indonesia. In the boreal zone, an increasing incidence of wildfire is also pushing up the carbon source from global forests (Kelly et al., 2013). The global soil organic matter pool is being reduced by agriculture; the soil organic carbon pool is typically reduced by half when native grassland vegetation is converted to agriculture (a function of both less input and faster decomposition associated with plowing).
Second, we are diverting 25–40 percent of the global terrestrial net primary production (NPP; the carbon fixed by plants and turned into biomass) from its previous pathway to local consumers (animals, insects) and decomposers, and using it for human purposes such as food, fuel, and fiber (Krausmann et al., 2013; Vitousek, Mooney, Lubchenco, and Melillo, 1997b). Global land NPP is about 60 Pg of C per year, only a factor of five larger than anthropogenic carbon emissions. This detour of NPP routes much of the fixed carbon directly back to the atmosphere rather than through the soil carbon pool (figure 4.1), thus tending to further diminish that pool.
One of the earliest discoveries of Earth system science regarding the global carbon cycle was that the terrestrial biosphere is now gaining carbon, despite deforestation and agricultural intensification. This conclusion is based on a simple mass balance calculation (table 4.1). Only three numbers are required. First, we must know the total anthropogenic carbon emissions. The fossil fuel and cement manufacturing component can be reliably estimated because fossil fuel is so economically important. Land use change emissions are more uncertain, but are relatively small. The second number is the amount of carbon accumulating in the atmosphere. It can be readily estimated by knowing the volume of the atmosphere and the annual increase in CO2 concentration. Since the atmosphere is well mixed on annual time scales, its CO2 concertation can be determined confidently with relatively few (about 100) measurement sites. Lastly, we must know the net uptake of carbon by the ocean. This is harder to estimate: it includes (1) the “solubility pump” (Raven and Falkowski, 1999), which is largely driven by the CO2 concentration gradient between the atmosphere and the ocean; and (2) the “biological pump,” previously discussed in chapter 3. Oceanographers are making many relevant observations and thus can reasonably estimate on an annual basis the ocean carbon sink.
TABLE 4.1 The Contemporary Anthropogenic Global Carbon Budget
FLUX TYPE |
FLUX UNITS |
Sources (emissions to atmosphere) |
|
Fossil fuel and cement |
9.3 |
Land cover change/land use |
1.0 |
Total |
10.3 |
Sinks (uptake from atmosphere) |
|
Atmosphere |
4.5 |
Ocean |
2.6 |
Land (by difference) |
3.1 |
Total |
10.2 |
Note: Sources for these estimates and their uncertainties are covered in Le Quere et al. (2016). Flux units are petagrams of carbon (Pg C) per year. There is a 0.1 Pg C rounding error between total sources and total sinks.
Based on this mass balance approach, we are seeing a net uptake of carbon by the land in recent years (about 3.1 PgC per year). In the tropics, carbon accumulation in intact forests is compensating for losses from deforestation. In mid and high latitude forest ecosystems, forest inventories suggest that carbon is accumulating (Pan et al., 2011). However, the mechanisms of that accumulation are poorly understood (this is often referred to as the “missing carbon sink”). Some is from regrowth of forests associated with abandonment of marginal agricultural lands, as in the northeastern United States and in Russia. Wood is also accumulating in long-lived wood buildings. Other carbon sinks may be related to (1) enhancement of plant growth by the CO2 itself (a key input to photosynthesis), (2) enhancement of plant growth by improved water use efficiency associated with high CO2, (3) deposition of nitrogen (a common fertilizer and limiting plant nutrient) generated by the process of fossil fuel combustion, and (4) climate warming (Schimel et al., 2001).
Between the land and the ocean, only about a third to a half (depending on the year) of fossil fuel–generated carbon is accumulating in the atmosphere as a greenhouse gas (about 2.1 ppm [parts per million] per year). Remarkably, the proportion of emitted CO2 each year that remains in the atmosphere has been stable on average over several decades (approximately 45 percent; Ballantyne, Alden, Miller, Tans, and White, 2012), which implies an increasing ocean plus land sink to compensate for the increasing anthropogenic emissions (11.2 PgC per year in 2015 compared with 1.5 PgC per year in 1950).
Both the land sink and the ocean sink are considered vulnerable. As the climate warms, we are seeing an increase in the area of forest burned each year in the boreal zone (van Lierop, Lindquist, Sathyapala, and Franceschini, 2015). These fires create both immediate CO2 emissions and long-term emissions associated with decay of dead wood. Land carbon sinks may decrease from other factors, including ozone pollution, drought, and poor land management (e.g., overgrazing). The ocean sink (per unit of CO2 increase) will decline as the ocean warms because of the simple physical fact that warm water holds less CO2 that cold water. Climate warming may also impact ocean NPP and the biological pump by way of influence on ocean circulation and stratification (Steinacher et al., 2010).
The net effect of human influences on the carbon cycle has been a rapid increase in the atmospheric CO2 concentration over the past 100 years (figure 4.2). The present concentration (2017) is approximately 410 ppm, compared with 260 ppm about 150 years ago as the Industrial Revolution accelerated, and 180 ppm about 15,000 years ago as Earth emerged from the last ice age (see figure 2.4). The current rate of increase in CO2 concentration (approximately 2 ppm per year) is unprecedented in geological history (Zeebe, Ridgwell, and Zachos, 2016) and appears to be rising. The CO2 concentration increase in 2015 was 3.05 ppm, the highest in the 56 years of measurements at Mauna Loa. As discussed in chapters 2 and 3, CO2 is an important greenhouse gas and the effect of the increasing CO2 concentration is an increasing strength of the atmosphere’s greenhouse effect, thus altering the global climate (IPCC, 2014a).
Another problematic aspect of the contemporary carbon cycle is the trajectory toward ocean acidification (figure 4.4; Feely, Doney, and Cooley, 2009). As the CO2 concentration in the atmosphere increases and more of it diffuses into the ocean, there is a shift in chemical equilibrium toward formation of a weak acid (carbonic acid), the product of interactions between CO2 and water molecules. The problem is that as the ocean becomes more acidic, the chemical reactions by which coral reefs are built, and by which some free-floating planktonic organisms grow their calcium carbonate shells, do not work as well. The ecology of marine plankton will hence be altered, including patterns of NPP. Like the rate of CO2 increase in the atmosphere, the rate of acidification of the surface ocean is likely unprecedented in geological history (because of the magnitude of the disequilibrium between the atmospheric and the whole ocean CO2 concentrations).
Methane (CH4) is next in importance after water vapor, CO2, and ozone in maintaining Earth’s background greenhouse effect (Kiehl and Trenberth, 1997). Its concentration has increased (figure 4.2) from 722 ppb (parts per billion) before 1750 to about 1834 ppb today, and it is second only to CO2 in contributing to the anthropogenic strengthening of the greenhouse effect (IPCC, 2014b). The cause of the increase in concentration is almost certainly anthropogenic, related to extensive rice cultivation (methanogens thrive in rice paddies), an increase in the population of domesticated ruminants that emit methane (e.g., cows), and leaks from the natural gas industry infrastructure. Natural sources are only about 40 percent of global methane emissions (GCP, 2017). Of special concern is the recent observation of increased methane emissions from the central United States (A. J. Turner et al., 2016). High latitudes are also receiving close attention because an increase there could signal the beginning of a significant positive feedback to global warming mediated by methane emissions from melting permafrost (Schuur et al., 2015).

FIGURE 4.4
The recent history of atmospheric CO2, ocean CO2, and ocean pH. Modified with permission from Feely et al. (2009).
As noted in chapter 1, Roger Revelle and Hans Suess characterized human alteration of the global carbon cycle as a large-scale geophysical experiment (Revelle and Suess, 1957). The recent dramatic increase in CO2 concentration is beginning to look a lot like a geological event comparable to some of the greenhouse gas–driven disruptions earlier in Earth’s history. For example, in the Paleocene–Eocene thermal maximum, CO2 and methane emissions increased, the CO2 concentration increased, the ocean acidified, and the marine biosphere was heavily impacted. How far we go along that path will be based on how long emissions continue and how strong carbon cycle feedbacks turn out to be.
NITROGEN
The global nitrogen (N) cycle differs from the carbon cycle in not having a mineral phase. Nitrogen cycles directly between the atmosphere (which is approximately 80 percent N2), and the biosphere. Biological fixation of nitrogen from the atmosphere into biomass is accomplished by specialized bacteria that may be either free living (in the soil or ocean) or symbiotic (as in lichens and soybeans). Smaller amounts of fixed nitrogen are transferred from the atmosphere by means of precipitation to the biosphere during lightening events. Decomposition of organic matter generally releases a form of nitrogen available for uptake by other organisms, thus driving the ecosystem loop of producer, consumer, and decomposer. Decomposition may also result in forms of nitrogen that are used by other specialized microbes as a substrate, and ultimately returned to the atmosphere.
Humanity has inserted itself into the global nitrogen cycle at several points (Vitousek et al., 1997a). In 1913, we began to fix nitrogen from the atmosphere into a plant-available form though an inorganic chemical process (the Haber–Bosch process). That chemical transformation requires a substantial input of energy, but the resulting fixed nitrogen has since been a boon to agriculture because crop production is often nitrogen limited. The background biological fixation of nitrogen at the global scale is approximately 170 TgN (teragrams of nitrogen) per year (1 Tg = 1012 g = 1,000,000,000,000 grams) and the input of plant-available nitrogen from lightening is much smaller (1–20 TgN per year). Through industrial processes we now fix approximately 100 TgN per year, and through cultivating crops such as soybeans we artificially fix about 25 TgN per year.
A second major pathway of human influence on the global nitrogen cycle is release of fixed nitrogen (i.e., not N2) to the atmosphere by combustion of fossil fuels. The magnitude of this flux is on the order of 25 TgN per year. The form of nitrogen produced by fossil fuel combustion is a molecule with one nitrogen atom and one or more oxygen atoms (NOx). Nitrate (an ionic form with three oxygen atoms) is very soluble (i.e., mixes well with water), and tends to be returned to the surface in precipitation as a dilute form of nitric acid. This form of nitrogen represents an artificial fertilization of unmanaged ecosystems and contributes to soil and aquatic acidification. Biogeochemists have developed the concept of nitrogen saturation at the ecosystem scale, referring to the level of artificial nitrogen deposition at which increased plant uptake cannot keep up, and deleterious effects begin to predominate (Aber et al., 1998).
In the United States and Europe, poorly regulated fossil fuel combustion in the mid-twentieth century led to high enough deposition of nitric and sulfuric acid (similarly produced) in precipitation to become a political issue. National and international agreements to scrub nitrogen and sulfur emissions from smokestacks and vehicles helped moderate the impacts on soils and water bodies (see chapter 8). Nevertheless, soil and aquatic acidification remain a significant environmental issue in the United States and Europe. This is even more of a concern in China and India, where fossil fuel combustion is still growing and associated nitrogen emissions are less regulated.
Combining nitrogen fixation from the fertilization industry, cultivation of nitrogen-fixing plants, and emissions from fossil fuel combustion yields a total of about 150 TgN per year, an amount of comparable magnitude to the background biological fixation. As noted earlier, one hypothesis for why the biosphere is currently sequestering carbon in some regions is that this massive subsidy of anthropogenic nitrogen is acting as a fertilizer in unmanaged ecosystems.
Another consequence of human intervention in the global nitrogen cycle is manifest by way of greenhouse gases. The nitrate form of nitrogen, besides being taken up by plants, can also be used by specialized microbes in their energy metabolism and transformed to nitrous oxide (N2O). This chemical form of nitrogen is a potent greenhouse gas, and its concentration in the atmosphere is increasing in recent years (figure 4.2), contributing 6 percent to current climate change (Lashof and Ahuja, 1990). Human activities contribute about 40 percent to the current global total annual N2O source. Nitrate-consuming microorganisms are most likely to capture nitrate when it is present in amounts beyond what plants need. Thus, the increase in N2O is very likely driven in part by excessive agricultural nitrogen fertilization.
Nitrogen oxides released by fossil fuel combustion also contribute to the formation of ozone in the lower atmosphere (the troposphere). Photochemical smog in large metropolitan areas is characterized by high ozone levels, and in sufficient concentration it is toxic to plants and animals. Like methane and nitrous oxide, ozone is a significant greenhouse gas and its increasing concentration in the lower atmosphere (the troposphere) is contributing to the total greenhouse gas–induced global warming.
PHOSPHORUS, SULFUR, AND CALCIUM
The global cycle of phosphorus (P) differs from the global cycles of carbon and nitrogen in not having a gaseous phase. Phosphorus becomes available to the terrestrial biosphere through chemical weathering of bedrock minerals. Humans currently mine and grind up rocks of high phosphorus content to produce phosphorus fertilizers for cropland.
The human-driven input of phosphorus to the biosphere is about triple the background delivery from mineral weathering (Smil, 2002). Natural phosphorus is not very soluble, so it tends to remain in place after being weathered out of minerals. It can be transported in streams and rivers to the ocean, where it is cycled by the ocean biota before sequestration in ocean sediments and incorporation into a mineral form.
Fertilizer phosphorus is delivered in a mobile form to facilitate uptake by crop plants. When supply and demand are not in synchrony or of the same magnitude, phosphorus tends to leach out of soil and, like nitrogen, contributes to eutrophication (excess productivity leading to oxygen depletion) in rivers and lakes. Deposition of phosphorus-rich human and animal wastes into water bodies exacerbates this problem. The high levels of phosphorus and nitrogen in large rivers that drain agricultural regions create so-called dead zones in coastal waters in some cases (Diaz and Rosenberg, 2008). At the mouth of the Mississippi River, a large dead zone forms each year for several months. During that period, the level of oxygen throughout the water column is too low to support fish and crustaceans.
The story of the global sulfur (S) cycle is similar in some respects to the nitrogen cycle in that the release of sulfur through combustion of fossil fuel (about 80 TgS per year) is large compared to background fluxes. Volcanic emissions to the atmosphere are only 14 TgS per year (Smil, 2002). Like nitrate, sulfate in the atmosphere from fossil fuel combustion precipitates out rapidly in an acidic form.
Sulfate molecules tend to aggregate and form aerosol particles in the atmosphere. These particles reflect solar radiation and become cloud condensation nuclei, and thus can have a significant cooling effect on the regional and global climate. The eruption of Mount Pinatubo in 1991 ejected enough sulfate into the atmosphere to cool global mean temperature 0.2°C–0.5°C for several years. Sulfate aerosols also influence ozone chemistry in the atmosphere and contribute to depletion of stratospheric ozone (Robock, 2000). Anthropogenic sulfate inputs to the atmosphere are currently having a significant cooling effect on global climate. However, the magnitude of the cooling effect is difficult to estimates because of the extreme spatial heterogeneity of the industrial emissions and the short atmospheric lifetime of sulfate—which is too short to be well-mixed throughout the earth’s atmosphere (Wilcox, Highwood, and Dunstone, 2013).
Other plant nutrients such as calcium, magnesium, and potassium are, like phosphorus, derived from rocks that are ground up for use in fertilizer. Also like phosphorus, they tend to be applied as fertilizer in a mobile form, and thus are susceptible to leaching. When irrigation is used improperly, these minerals (in fertilizer or brought to the surface with evaporative flow) are left as salts at the surface during periods of rapid soil evaporation. This salinization is a significant form of soil degradation. Poorly managed irrigation and associated salinization has made approximately 10 percent of globally irrigated land unusable for agricultural purposes, and the area of degraded land is increasing (McNeill, 2000).
METALS
The rapid evolution of the technosphere has depended on our ability to isolate and manipulate elements such as gold, copper, iron, and aluminum. These elements were isolated from rocks, purified, and combined in alloys in ever-increasing amounts over the course of human history. Examination of the global budgets for many of these metals suggests that humans mobilize as much or more than the background fluxes (Rauch and Pacyna, 2009).
Of particular interest with respect to the biosphere are those elements that are toxic at low concentrations. Lead began to be added to gasoline beginning in the 1920s to increase automobile engine performance and life. However, based on accumulating evidence of its impacts on human health (neurotoxicity), its use as an additive was halted, resulting in a large decrease in anthropogenic emissions to the atmosphere. A decline to virtually preindustrial concentrations shows up nicely in ice cores from the Greenland ice sheet (Boutron, 1995).
Mercury (Hg) is also toxic and is added to the atmosphere by way of fossil fuel combustion (mostly coal) and to water bodies by way of industrial applications (mining). Global anthropogenic mercury emissions are at least an order of magnitude greater than natural emissions (Nriagu, 1989) and only 17 percent of the mercury in the contemporary ocean is of natural origin (Amos, Jacob, Streets, and Sunderland, 2013). Because of its tendency to bioaccumulate, mercury can reach toxic concentrations in freshwater and marine fish. As with lead, there has been enough research concerning its health effects on humans and wildlife to justify tighter regulation of mercury emissions from power plants (in some countries).
Other trace metals, or heavy metals, that are potentially toxic and are now being released into the environment in increasing quantities include cadmium and chromium. They are extracted from rocks for industrial purposes. Recently there has been increasing concern in China with movement of cadmium from mining wastes into freshwater, and then into the human food chain by way of irrigated crops (Hsu and Miao, 2014).
RADIOACTIVE ELEMENTS
Radioactive atoms spontaneously emit high-energy electromagnetic radiation and particles (e.g., electrons) during decay from one element form to another. These atoms originated in nuclear fusion reactions, mostly in stars. Radioactive uranium was concentrated in Earth’s core as the planet solidified, and energy from its decay and that of other radioactive materials drives the tectonic cycles discussed in chapter 2.
Scientists first discovered radioactivity in the context of X-rays in the late nineteenth century. Physicists gradually worked out the processes by which radioactive atoms were formed and decay. Besides fusion reactions in stars, they could form in the atmosphere by way of interactions of atoms with high-energy solar radiation or cosmic radiation (from outside the solar system). By World War II, physicists realized the potential for explosive chain reactions if radioactive atoms were concentrated. The successful construction of atomic bombs helped end World War II, but also set off a nuclear arms race associated with the subsequent Cold War.
Aboveground nuclear testing in the 1940s and 1950s pushed up the atmospheric burden of a variety of short-lived and long-lived radioactive species. Experiments with animals had shown that inhaled or ingested radioactive atoms can induce various forms of cancer. The realization that aboveground nuclear testing was a significant health threat prompted an international agreement to curtail it (see chapter 8). The atmospheric concentrations of bomb-generated radioactive materials have declined, but the long-lived chemical species originating in bomb testing can still be detected in soil.
The nuclear power industry has also been a source of radioactive species to the environment. Europe became aware of the nuclear power plant accident in Chernobyl when radioactivity detectors began lighting up as the radioactive fallout blew in from the east. The sites around nuclear power plant accidents such as Chernobyl and Fukushima are heavily contaminated with radioactive material and must be closed to human habitation.
OXYGEN
Earth’s atmosphere is 20.8 percent oxygen (O2). We’ve noted that James Lovelock formulated the Gaia hypothesis in part because there was so much oxygen in the atmosphere despite it being quite chemically reactive. The source of most oxygen in the atmosphere is the splitting of water molecules during photosynthesis. The primary sink of oxygen is metabolic respiration, in which oxygen reacts with energy-rich organic compounds (e.g., sugars). Water is a by-product of aerobic respiration; hence oxygen cycles between the atmosphere, the biosphere, and hydrosphere. An alternate oxygen sink is combustion of biomass (i.e., fire).
By combustion of fossil fuels, the technosphere has boosted the consumption of oxygen and correspondingly the production of CO2 (hence global warming). However, since the concentration of oxygen is orders of magnitude greater than that of CO2, there has been minor impact on the oxygen concentration in the atmosphere. Ralph Keeling, son of David Keeling (who first detected the increase in atmospheric CO2) devised an oxygen-monitoring instrument sensitive enough to detect the slight decreasing oxygen trend in recent decades that corresponded to the CO2 increase (and an annual cycle linked to the annual oscillation of the CO2 concentration). Earth system scientists have no concerns about oxygen depletion from fossil fuel combustion, or possible reduction in oxygen production because of human impacts on global photosynthesis. There is, however, concern about declining oxygen in the ocean, associated with climate warming, and possible biological impacts (R. F. Keeling, Kortzinger, and Gruber, 2010).
Atmospheric chemists also have concerns about two more subtle aspects of oxygen chemistry. The more familiar issue is stratospheric ozone depletion (Rowland, 2006). The subtler issue involves hydroxyl radical depletion (IPCC, 2007). Both issues center on the extraordinarily complicated chemistry of oxygen in the atmosphere, one of the more formidable topics in Earth system science.
We have noted the role of ozone (O3) as a greenhouse gas but it is also important to the Earth system because in the stratosphere it absorbs ultraviolet (UV) wavelengths of solar radiation, in effect protecting the biosphere from this highly energetic form of sunlight. A “hole” in the stratospheric ozone layer was discovered in the 1980s when scientists concluded that instruments in Antarctica that had been monitoring the column abundance of ozone and recording anomalous drops during the winter since the 1970s were not faulty, but were registering accurate numbers after all. Indeed, the level of ozone in the stratosphere during the polar winter was much lower than had been previously measured. Atmospheric scientists had already identified a possible mechanism of ozone depletion involving chemical reactions driven by anthropogenically created chlorofluorocarbons (CFCs). These compounds were developed in the 1930s for use as nontoxic, nonflammable refrigerants and propellants. Their inert nature made them ideal for those purposes, but an unintended consequence was that they accumulated in the atmosphere. After slow diffusion to the high-energy environment of the stratosphere, they broke down, and the chlorine became chemically reactive as a catalyst to ozone consumption. Here, clearly, the technosphere was threatening the global life support system.
It took the atmospheric chemistry community a decade or so to understand what was causing stratospheric ozone depletion, then more years to convince the policy community to do something about it. But with the ratification of the Montreal Protocol (discussed in chapter 8), the industrial nations of the world agreed to stop CFC production. The treaty has largely been effective, and the CFC concentration is slowly decreasing (figure 4.5), with an associated shrinking of the ozone hole. The only downside to the story is that the replacement refrigerants are also long-lived compounds that accumulate in the atmosphere, and they are strong greenhouse gases.

FIGURE 4.5
Time series for effective equivalent chlorine concentration (a metric related to capacity to deplete stratospheric ozone); ppb = parts per billion. Adapted from NOAA (http://www.esrl.noaa.gov/gmd/hats/graphs/graphs.html).
The hydroxyl radical (OH) is the so-called cleanser of the atmosphere. It is a very short-lived product of photochemical reactions involving oxygen. OH attacks a wide variety of reactive carbon and nitrogen molecules and converts them to forms that leave the atmosphere through precipitation. The potential problem is that the technosphere is loading up the atmosphere with so many reactants (pollutants), that the availability of OH may be decreasing. Over half the global emissions of reactive carbon and nitrogen compounds are now anthropogenic in origin (Lelieveld, Dentener, Peters, and Krol, 2004). The consequence of high anthropogenic emissions of pollutants is that the atmospheric lifetime of molecules such as methane (a greenhouse gas) is extended. The complexity of the atmospheric chemistry and the extremely brief lifetime of the OH radical molecule keep this an active area of Earth system science research.
THE HYDROLOGIC CYCLE
Humans are master manipulators of water. We now use in one way or another approximately 50 percent of the global freshwater flow in streams and rivers (Goodie, 2006; McNeill, 2000). Some of that water is returned to the river from which it was appropriated (e.g., downstream from large cities), but often in a polluted form.
Large dams and associated irrigation are now found on almost all major river systems on the planet and have the effect of increasing evaporation, which reduces downstream river flow. The Aral Sea in Central Asia has virtually disappeared as of result of diverted inflows. Large river deltas all over the planet are starved of silt by upriver dams, and subject to salt water intrusion by reduced flows.
Another indirect human influence on stream flow is a shift in the pattern in spring runoff. As the climate warms (from increasing greenhouse gas emissions) in mountainous area, more precipitation falls as rain rather than snow, consequently ending up in stream flow earlier (Barnett et al., 2008). What snow does fall also tends to melt earlier, thus providing less of a sustained stream flow during the growing season. Glaciers are in retreat globally (NSIDC, 2013) and it is anticipated that their demise will have significant impacts on seasonal availability of water in several heavily populated regions, such as the Ganges River Basin in India.
In some regions, the quantity of water extracted from rivers is augmented by significant amounts from underground aquifers. Depending on depth, belowground aquifers are naturally recharged, but often at nothing like the rates at which water is now being extracted. The deepest aquifers are essentially fossil water, and these are largely being mined rather than used sustainably (Pearce, 2006).
The story of the Ogallala Aquifer in the United States is a great case study. This underground reservoir underlies much of Nebraska and stretches south to western Kansas, Oklahoma, and Texas. Extraction is largely unregulated, and wells feeding center point irrigation agriculture have proliferated in recent decades. The aquifer is being rapidly emptied in some areas; consequently, withdrawals have been curtailed by the requirement to dig ever deeper wells. A similar story is unfolding in the Central Valley of California and in the Indus River Basin in India. Remarkably, there is now a satellite-borne sensor (GRACE, further discussed in chapter 9) that can monitor the size of groundwater reservoirs based on faint signals in the gravitational field (Strassberg, Scanlon, and Chambers, 2009).
Besides water on the surface and in the ground, we are influencing the amount of water in the atmosphere by extensive changes in land cover. Deforestation reduces the rate of water return to the atmosphere via transpiration and changes the local energy balance. In Costa Rica, deforestation over large areas in the eastern coastal plain is resulting in higher clouds and a drying of the downwind mountain cloud forests (U. S. Nair, Lawton, Welch, and Pielke, 2003). In the Amazon Basin, regional climate model simulations suggest that continued deforestation will set up a positive feedback loop such that large areas of deforested land will reduce local precipitation and cause degradation of adjacent intact rain forests (Costa and Foley, 2000).
At the global scale, the warming of the atmosphere associated with human-induced rise in the concentrations of various greenhouse gases is increasing the amount of water vapor (another greenhouse gas) held in the atmosphere. Since warm air holds more water than cooler air, and we live on a planet whose surface is two thirds water, there is increased evaporation into the atmosphere over water as climate warms (Chung, Soden, Sohn, and Shi, 2014). This positive feedback to climate warming was noted in chapter 3.
More water vapor leads to an associated increase in global precipitation. However, that overall increase is playing out as big increases in some areas and decreases in other areas. One pattern that is both predicted by the general circulation climate models that simulate the effects of rising greenhouse gas concentration, and is beginning to be observed by networks of meteorological stations, is an ongoing drying of the lower mid latitudes (e.g., Neelin, Munnich, Su, Meyerson, and Holloway, 2006; Seager et al., 2007). This drying trend may already be having significant impacts on vegetation (Breshears et al., 2005).
A quite pervasive but poorly understood indirect human influence on the hydrologic cycle is the increasing water use efficiency of vegetation (Keenan et al., 2013). In chapter 2, we noted that one of the paleo-proxies for atmospheric CO2 concentration is stomatal density in fossilized leaves. Plants tend to reduce stomatal density as CO2 concentration increases because diffusion of CO2 through the stomata is faster. A key benefit of low stomatal density is less evaporation of water vapor out of the leaf (i.e., transpiration), and therefore less sensitivity to drought. The effect on transpiration at the canopy scale is difficult to predict, however, because vegetation may also increase leaf area (Tng et al., 2012). The effect of reduced transpiration at the watershed scale is local warming, because solar radiation is absorbed and reradiated rather than being used for transpiration. Less transpiration could also potentially result in an increase in stream flow (A. J. Turner et al., 2016; Wiltshire et al., 2013).
Lastly, let’s consider changes in the ocean. The approximately 1°C increase in global mean temperature over the past century has already significantly influenced sea level. Satellite monitoring of glaciers and ice sheets has documented a global decline in volume that explains part of the increase. Thermal expansion as the ocean warms explains the rest. The current rate of sea level rise is approximately 3 mm (millimeters) per year, and the rate is accelerating (IPCC, 2014b, Watson, 2016). The total increase since 1901 is approximately 200 mm (close to 8 inches). The impacts of sea level rise on the technosphere are limited at this point, but there is every reason to believe that we are at the very beginning of a rise in sea level that will amount to many meters and play out over hundreds of years (see chapter 6).
We are also altering the ocean’s thermohaline circulation (THC). This so-called “ocean conveyor belt” brings warmth to high latitudes in the North Atlantic. The underlying mechanism is based on flow of the Gulf Stream current along the east coast of North America, which is partially driven by the Atlantic Ocean component of the THC (the Atlantic meridional overturning circulation). This flow keeps Europe warmer than would be the case without it (Palter, 2015). Typically, as water in the Gulf Stream cools at high latitude, it becomes denser and sinks, which maintains the flow of the THC (Broecker, 1987). However, with global warming–induced melting of the Greenland ice cap, and possibly more precipitation because of the intensification of the hydrologic cycle (Huntington, 2006), more freshwater is input to the North Atlantic, causing a freshening of those waters, and hence a decrease in density and less propensity to sink (Rahmstorf et al., 2015). In addition, the disproportionate heating of the polar regions by greenhouse gas warming reduces the north-south temperature gradient between the tropics and the high latitudes, which diminishes the Gulf Stream. The impacts of a slowed THC include a cooler Europe and sea level rise along the east coast of North America.
ESSENTIAL POINTS
The technosphere juggernaut is rapidly altering many of the global biogeochemical cycles. Vernadsky was certainly prescient in recognizing as early as the 1920s that humanity is the equivalent of a new geological force. Since the 1950s, especially, there has been rapid acceleration of mass fluxes through the technosphere and into the atmosphere, biosphere, and hydrosphere. Besides altering the flux rates of many background biogeochemical cycling reactions, the technosphere has brought to Earth’s surface (our biosphere) high levels of toxic elements such as cadmium, and broadly distributed radioactive elements such as cesium and uranium. Environmental problems arise when the background mechanisms of biogeochemical recycling are overwhelmed (or do not exist) and undesirable waste products accumulate. Technosphere impacts include a rapid increase in the atmospheric concentrations of the most important greenhouse gases, and intensification of the hydrologic cycle.
IMPLICATIONS
1. The reflexive consciousness of Homo sapiens (thinking matter, if you will) is a qualitatively new addition to the several billion-year-old biosphere (living matter). The capacity of humans to work collectively in large social groups, and to think in a scientific manner, has led to a massive disruption of the global biogeochemical cycles. The same brainpower and technological prowess that has induced a crisis in natural resource management must now devise a more sustainable relationship of technosphere to biosphere (the focus of chapters 7–10).
2. The growth of the technosphere is inducing a warming of the global climate. For now, the reciprocal impacts of that climate change on the technosphere have been minor. However, at some point we may see a negative feedback loop emerge such that global environmental change begins to inhibit the growth of the technosphere.
3. The current geologically unprecedented rate of increase in atmospheric CO2 is inducing rapid acidification of ocean surface waters. This change in ocean chemistry is beginning to alter ocean productivity and the ocean carbon sink. Thus, there are two compelling reasons (climate change and ocean acidification) to rapidly reduce fossil fuel carbon emissions.
4. Sulfur emissions associated with fossil fuel combustion produce sulfate aerosols with a significant cooling influence on the current climate. When burning of fossil fuels is constrained, some additional warming will be manifest because of the reduction in sulfate aerosols. This additional warming must be planned for.
FURTHER READING
Archer, D. (2010). The global carbon cycle. Princeton, NJ: Princeton University Press.
McNeill, J. R. (2000). Something new under the sun. New York: Norton.