The concept of energy plays an important role in all of the sciences. In chemistry, all physical and chemical changes have energy considerations associated with them. To understand how and why these changes happen, an understanding of energy is required.
Energy is defined as the capacity to do work or heat things. Work is done whenever a force is applied over a distance. Therefore, anything that can force matter to move, to change speed, or to change direction has energy. Heat is the flow of energy due to temperature differences. The following example will help you understand this definition of energy.
Raising a hammer above a nail about to be pounded into a block of wood stores energy in the hammer. Energy is also stored in the muscles of the arm that has lifted the hammer. When the hammer is brought down to impact upon the nail, the nail will likely be forced to move into the wood and so work is done on the nail. Continuing to pound on the nail will force it to move even farther into the wood but may also result in noticeable heating of the nail. If the nail is unable to move farther into the wood upon additional pounding (perhaps because the wood is very hard), the only thing that will occur is that the nail will heat up. In other words, upon touching the nail with your cooler fingers, the nail will be able to transfer energy to you with a greater capacity than it previously had before being pounded.
Typical units for energy include the joule (J) and the calorie (cal). Each could be used to describe either aspect of energy. However, work is generally reported in joules or kilojoules (kJ) while heat is often given in calories or kilocalories (kcal). The joule is a smaller unit of energy than the calorie, though, with the relationship being 4.184 J = 1 calorie.
Energy may appear in a variety of forms. Most commonly, energy in reactions is evolved as heat. Some other forms of energy are light, sound, mechanical energy, electrical energy, and chemical energy. Energy can be converted from one form to another, as when the heat from burning fuel is used to vaporize water to steam. The energy of the steam is used to turn the wheels of a turbine to produce mechanical energy. The turbine turns the generator armature to produce electricity, which is then available in homes for use as light or heat, or in the operation of many modern appliances.
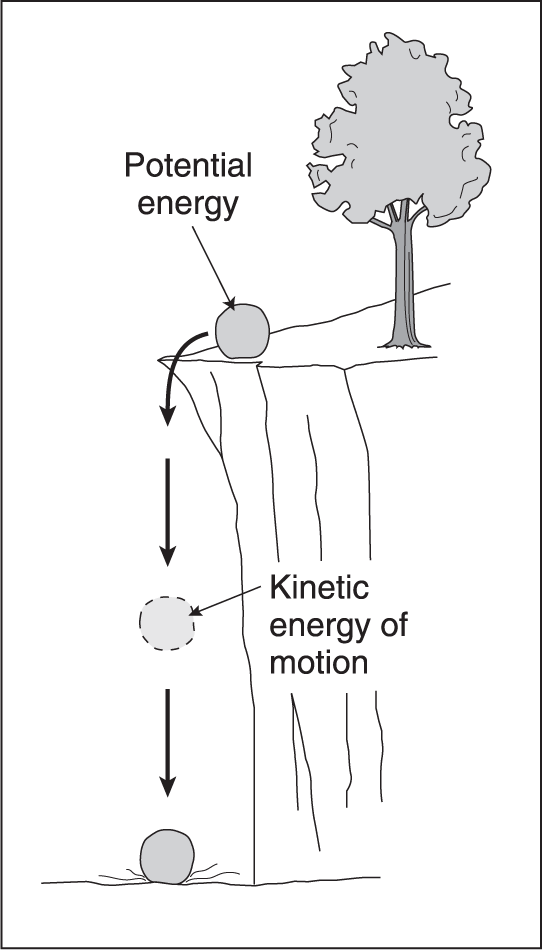
Two general types of energy are potential energy and kinetic energy. Potential energy is stored energy due to overcoming forces in nature. Kinetic energy is energy of motion. In other words, by virtue of the fact that matter is moving, it possesses the ability to move other things or to heat them. Potential energy can be converted into kinetic energy by allowing the forces that were overcome to be applied. Consequently, the gained kinetic energy associated with the substance has the same capacity to move or heat things as the substance's original, but now lost, potential energy. In other words, energy is conserved. These concepts are illustrated in Figure 1.3 by considering a boulder sitting on the side of a mountain. It has high potential energy due to its position above the valley floor since the boulder had to overcome gravity to rest there. If it falls, however, its potential energy is converted to kinetic energy and will move or heat up the matter with which it collides. This illustration is very similar to the situation of electrons cascading to lower energy levels in the atomic model described in Chapter 2.

When physical or chemical changes occur, the total capacity of the substances to exchange heat changes. The total capacity a substance has to exchange heat has a name, enthalpy, and is symbolized by the letter H. Since enthalpy changes as physical or chemical processes unfold, chemists typically investigate the change in this property, which is symbolized by ΔH. The equation is:
If the enthalpy of the products is greater than the enthalpy of the reactants, ΔH is a positive quantity (ΔH > 0) and the reaction is endothermic. If, however, the enthalpy of the products is less than the enthalpy of the reactants, ΔH is a negative quantity (ΔH < 0) and the reaction is exothermic. This relationship is shown graphically in Figures 1.4 and 1.5. This topic is developed in detail in Chapter 8.
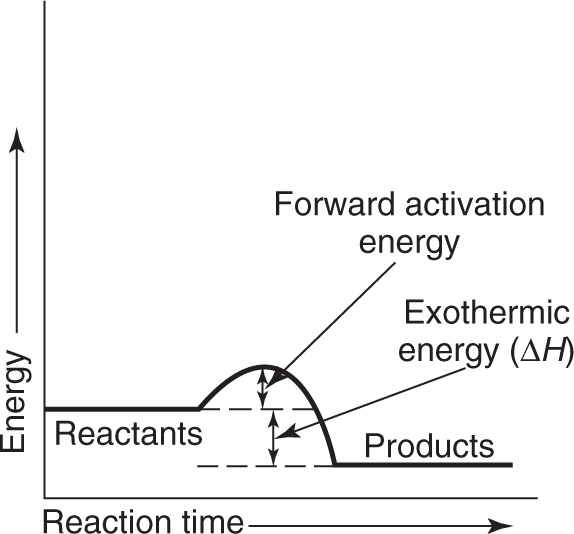
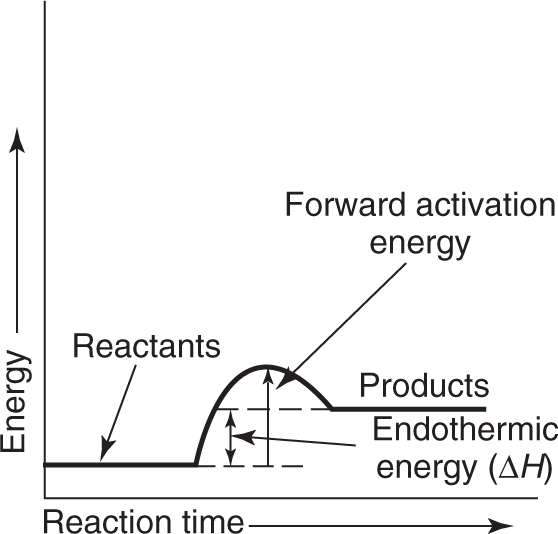
Notice that in Figures 1.4 and 1.5, the term activation energy is used. The activation energy is the energy necessary to get the reaction going by increasing the energy of the reactants so they can combine. You know you have to heat paper before it burns. This heat raises the energy of the reactants so that the burning can begin; then enough energy is given off from the burning so that an external source of energy is no longer necessary.
Experiments have shown that energy is neither gained nor lost in physical or chemical changes. This principle is known as the Law of Conservation of Energy and is often stated as follows: Energy is neither created nor destroyed in ordinary physical and chemical changes. If the system under study loses energy, the reaction is exothermic and the ΔH is negative. Therefore, the system’s surroundings must gain the energy that the system loses so that energy is conserved.