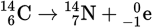
A helpful application of radioactive decay is in the determination of the ages of substances such as rocks and relics that have bits of organic material trapped in them. Because carbon-14 has a half-life of about 5,700 years and occurs in the remains of organic materials, it has been useful in dating these materials. A small percentage of CO2 in the atmosphere contains carbon-14. The stable isotope of carbon is carbon-12. Carbon-14 is a beta emitter and decays to form nitrogen-14:
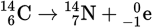
In any living organism, the ratio of carbon-14 to carbon-12 is the same as in the atmosphere because of the constant interchange of materials between organism and surroundings. When an organism dies, this interaction stops, and the carbon-14 gradually decays to nitrogen. By comparing the relative amounts of carbon-14 and carbon-12 in the remains, the age of the organism can be established. Carbon-14 has a half-life of 5,700 years. If a sample of wood had originally contained 5 grams of carbon-14 and now had only half or 2.5 grams of carbon-14, its age would be 5,700 years. In other words, the old wood emits half as much beta radiation per gram of carbon as that emitted by living plant tissues. This method was used to determine the age of the Dead Sea Scrolls (about 1,900 years) and has been found to be in agreement with several other dating techniques.