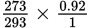Practice Exercises
Record your answers on a separate sheet of paper.
-
The most abundant element in Earth’s crust is
- sodium
- oxygen
- silicon
- aluminum
-
A compound that can be decomposed to produce oxygen gas in the lab is
- MnO2
- NaOH
- CO2
- KClO3
-
In the usual laboratory preparation equation for the reaction in question 2, what is the coefficient of O2?
- 1
- 2
- 3
- 4
-
In the graphic representation of the energy contents of the reactants and the resulting products in an exothermic reaction, the energy content would be
- higher for the reactants
- higher for the products
- the same for both
- impossible to determine
-
The process of separating components of a mixture by making use of the difference in their boiling points is called
- destructive distillation
- displacement
- fractional distillation
- filtration
-
When oxygen combines with an element to form a compound, the resulting compound is called
- a salt
- an oxide
- oxidation
- an oxalate
-
According to the activity chart of metals, which metal would react most vigorously in a dilute acid solution?
- zinc
- iron
- aluminum
- magnesium
-
Graham’s Law refers to
- boiling points of gases
- gaseous diffusion
- gas compression problems
- volume changes of gases when the temperature changes
-
When 200 milliliters of a gas at constant pressure is heated, its volume
- increases
- decreases
- remains unchanged
-
When 200 milliliters of a gas at constant pressure is heated from 0°C to 100°C, the volume must be multiplied by
- 0/100
- 100/200
- 273/373
- 373/273
-
If you wish to find the corrected volume of a gas that was at 20°C and 1 atmosphere pressure and conditions were changed to 0°C and 0.92 atmosphere pressure, by what fractions would you multiply the original volume?
-
-
When the level of mercury inside a gas tube is higher than the level in the reservoir, you find the correct pressure inside the tube by taking the outside pressure reading and ? the difference in the height of mercury.
- subtracting
- adding
- dividing by 13.6
- doing both (C) and (A)
-
If water is the liquid in question 12 instead of mercury, you can change the height difference to an equivalent mercury expression by
- dividing by 13.6
- multiplying by 13.6
- adding 13.6
- subtracting 13.6
-
Standard conditions are
- 0°C and 14.7 mm
- 273 K and 760 mm Hg
- 273°C and 760 mm Hg
- 4°C and 7.6 mm Hg
-
When a gas is collected over water, the pressure is corrected by
- adding the vapor pressure of water
- multiplying by the vapor pressure of water
- subtracting the vapor pressure of water at that temperature
- subtracting the temperature of the water from the vapor pressure
-
At 5.00 atmospheres pressure and 70°C how many moles are present in 1.50 liters of O2 gas?
- 0.036
- 0.103
- 0.266
- 0.536
Directions: Every set of the given lettered choices below refers to the numbered questions immediately below it. For each numbered item, choose the one lettered choice that fits it best. Every choice in a set may be used once, more than once, or not at all.
-
-
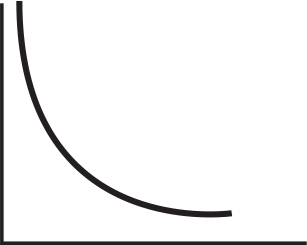
Questions 17–20 refer to the following graphs, assuming that the axes on each are appropriately labeled:
-
-
Which is a graphic depiction of Boyle’s Law?
-
Which is a graphic depiction of Charles’s Law?
-
Which is a graphic depiction of the relationship of the pressure of a given volume of gas with the absolute temperature?
-
Which graph shows the distribution of molecules with respect to their kinetic energy at different temperatures?
-