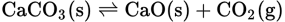
The examples so far have involved systems made up of only gaseous substances. Expression of the K values of systems changes when other phases are present.
If the experimental data for this reaction are studied:
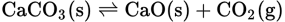
it is found that at a given temperature an equilibrium is established in which the concentration of CO2 is constant. It is also true that the concentrations of the solids have no effect on the CO2 concentration as long as both solids are present. Therefore, the Keq, which would conventionally be written like this:
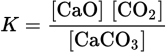
can be modified by incorporating the concentrations of the two solids. This can be done since the concentration of solids is fixed. It becomes a new constant K, known as:

Any heterogeneous reaction involving gases does not include the concentrations of pure solids. As another example, K for the reaction

is

When a weak acid does not ionize completely in a solution, an equilibrium is reached between the acid molecule and its ions. The mass action expression can be used to derive an equilibrium constant, called the acid dissociation constant, for this condition. For example, an acetic acid solution ionizing is shown as

The concentration of water in moles/liter is found by dividing the mass of 1 liter of water (which is 1,000 g at 4°C) by its gram-molecular mass, 18 grams, giving H2O a value of 55.6 moles/liter. Because this number is so large compared with the other numbers involved in the equilibrium constant, it is practically constant and is incorporated into a new equilibrium constant, designated as Ka. The new expression is

Ka incorporates the concentration of water.
Ionization constants have been found experimentally for many substances and are listed in chemical tables. The ionization constants of ammonia and acetic acid are about 1.8 × 10−5. For boric acid Ka = 5.8 × 10−10, and for carbonic acid Ka = 4.3 × 10−7.
If the concentrations of the ions present in the solution of a weak electrolyte are known, the value of the ionization constant can be calculated. Also, if the value of Ka is known, the concentrations of the ions can be calculated.
A small value for Ka means that the concentration of the un-ionized molecule must be relatively large compared with the ion concentrations. Conversely, a large value for Ka means that the concentrations of ions are relatively high. Therefore, the smaller the ionization constant of an acid, the weaker the acid. Thus, of the three acids referred to above, the ionization constants show that the weakest is boric acid, and the strongest, acetic acid. It should be remembered that, in all cases where ionization constants are used, the electrolytes must be weak in order to be involved in ionic equilibria.
Because water is a very weak electrolyte, its ionization constant can be expressed as follows:
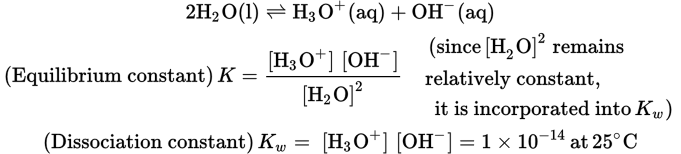
Kw incorporates the [H2O]2.
From this expression, we see that for distilled water [H3O+] = [OH−] = 1 × 10−7. Therefore, the pH, which is −log[H3O+], is
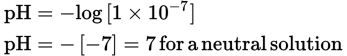
The pH range of 1 to 6 is acid, and the pH range of 8 to 14 is basic. See Figure 10.2 below.

Know the meaning of the pH scale.
A saturated solution of a substance has been defined as an equilibrium condition between the solute and its ions. For example:

The equilibrium constant would be:

Ksp incorporates the concentration of the solute.
Since the concentration of the solute remains constant for that temperature, the [AgCl] is incorporated into the K to give the Ksp, called the solubility constant:

This setup can be used to solve problems in which the ionic concentrations are given and the Ksp is to be found or the Ksp is given and the ionic concentrations are to be determined.