Answers and Explanations
-
(C) Because carbon has 4 electrons in its outer energy level, it usually forms four covalent bonds to fill each of four sp3 orbitals.
-
(D) Bituminous coal has too much gaseous impurities to burn at a high temperature needed to refine iron ore. It is heated in coke ovens to form the hotter and cleaner burning coke.
-
(C) The reaction of an acid and a carbonate is the usual way to prepare CO2.
-
(D) The reaction is: CO2 + Ca(OH)2 → CaCO3↓ + H2O.
-
(B) The lead in a lead pencil is a mixture of graphite and clay.
-
(B) The first alkane is methane, CH4.
-
(D) The slight oxidation of a primary alcohol produced an aldehyde.
An example is:
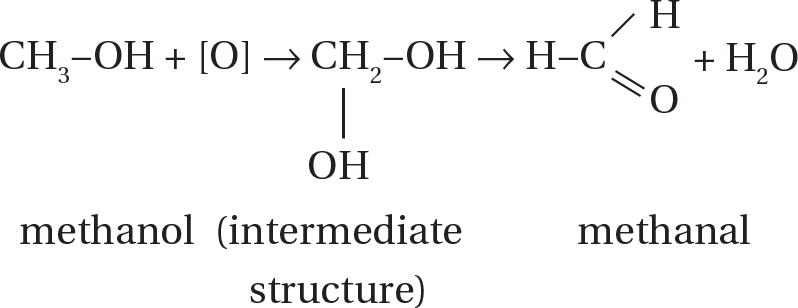
-
(E) The functional group for an ester is shown as –COO–.
-
(A) Ethanol can be oxidized into the organic acid enthanoic acid, which has the common name of acetic acid.
-
(C) The formation of an ester is from the reaction of an organic acid and an alcohol. The general equation is:
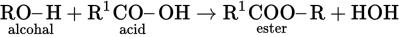
-
(B) Isomers are compounds that have the same composition but differ in their structural formulas.
-
(D) Ethene is the first member of the alkene series that has one double bond. Because it has a double bond, it is said to be unsaturated. The alkane series, which has all single bonds between the carbon atoms in the chain, is called a saturated series.
-
(E) The NH2− group, called an amine group, makes this structure ethylamine. This type of organic structure is called an amide.
-
(C) The methyl (CH3–) group and the propyl (–C3H7) group attached to a center oxygen (–O–) makes this methyl propyl ether (methoxypropane).
-
(A) Propane is the third member of the alkane series, which is made up of a chain of single-bonded carbons and hydrogens with the general formula of CnH2n+2.
-
(B) Ethanoic acid is composed of a methyl group attached to the carboxyl group (–COOH). The latter is the functional group for an organic acid.
-
(D) The propan- part of the name tells you its basic structure is from propane, which is a three-carbon alkane. The –one part tells you it is a ketone that has a double-bonded oxygen attached to the second carbon in the chain.
-
(B) The ethanoic acid contains the carboxyl group (–COOH) that is shown in (B). This is the identifying functional group for organic acids.
-
(D) The functional group for ketones is a carbon in the chain double bonded to an oxygen atom (
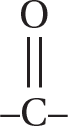 ). The number in the front tells you which carbon has the double-bonded oxygen attached to it.
). The number in the front tells you which carbon has the double-bonded oxygen attached to it. -
(E) The amine group is the nitrogen with two hydrogens, (–NH2), attached to a chain carbon. These are basic to the amino acid structures in the body.