Practice Exercises
Record your answers on a separate sheet of paper.
-

In the reaction setup shown above, which of the following are true?
- This setup can be used to prepare a soluble gas by water displacement.
- This setup involves a decomposition reaction if the substance heated is potassium chlorate.
- This setup can be used to prepare an insoluble gas by water displacement.
- I only
- II only
- I and III
- II and III
- I, II, and III
-
Questions 2–4 refer to the following diagram:

- Around the thermometer
- In the condenser
- In the circulating water
- In the heated flask
- In the distillate
-
In this laboratory setup for distillation, where does the vaporization take place?
-
If the liquid being distilled contains dissolved magnesium chloride, where will it be found after distillation is completed?
-
If the liquid being distilled contains dissolved ammonia gas, where will it be found after distillation is completed?
-
If the flame used to heat a flask is an orange color and blackens the bottom of the flask, what correction should you make to solve this problem?
- Move the flask farther from the flame.
- Move the flask closer to the flame.
- Allow less air into the collar of the burner.
- Allow more air into the collar of the burner.
- The problem is in the supply of the gas, and you cannot fix it.
-
Questions 6–8 refer to the following diagram:
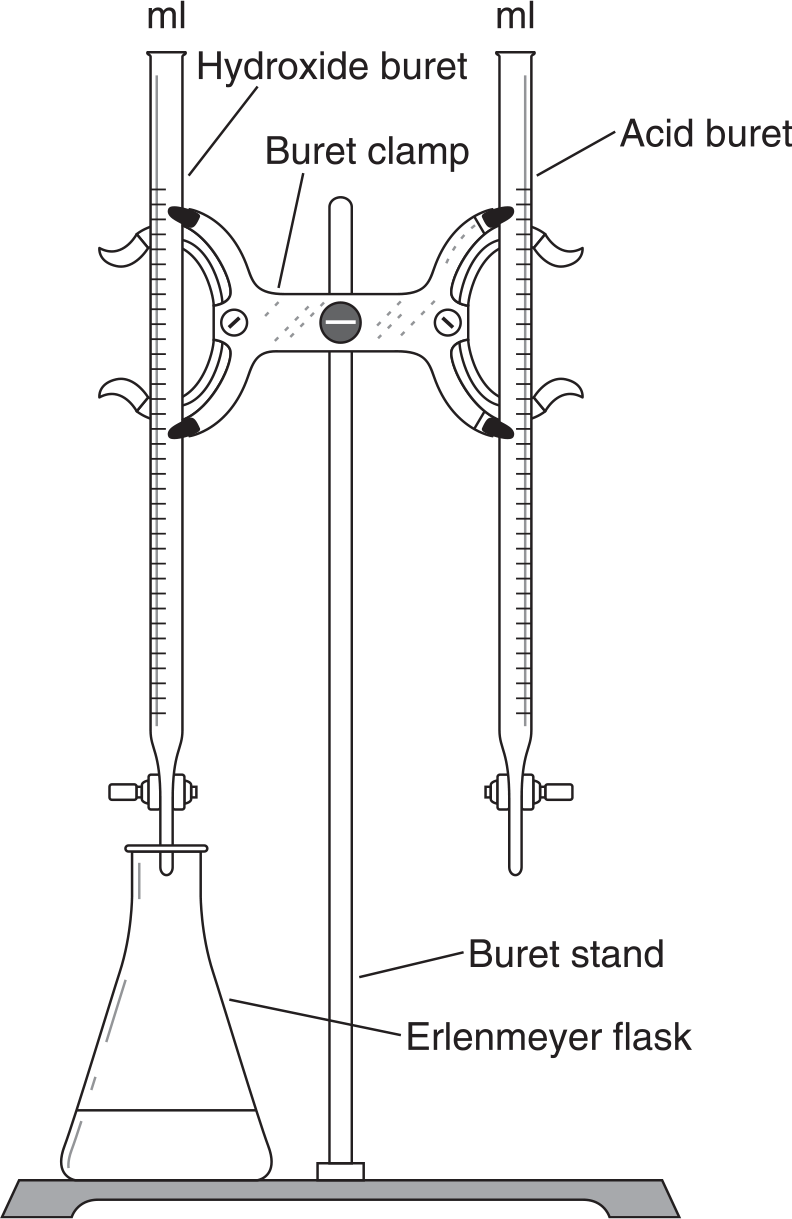
-
In the above titration setup, if you introduce 15 mL of the NaOH with an unknown molarity into the flask and then add 5 drops of phenolphthalein indicator, what will you observe?
- A pinkish color will appear throughout the solution.
- A blue color will appear throughout the solution.
- There will be a temporary pinkish color that will dissipate.
- There will be a temporary blue color that will dissipate.
- There will not be a color change.
-
If the HCl is 0.1 M standard solution and you must add 30 mL to reach the end point, what is the molarity of the NaOH?
- 0.1 M
- 0.2 M
- 0.3 M
- 1 M
- 2 M
-
When is the end point reached and the volume of the HCl recorded in this reaction?
- When the color first disappears and returns in the flask.
- When equal amounts of HCl and NaOH are in the flask.
- When the color disappears and does not return in the flask.
- I only
- III only
- I and III
- II and III
- I, II, and III
-
Questions 9–11 refer to the following diagram:
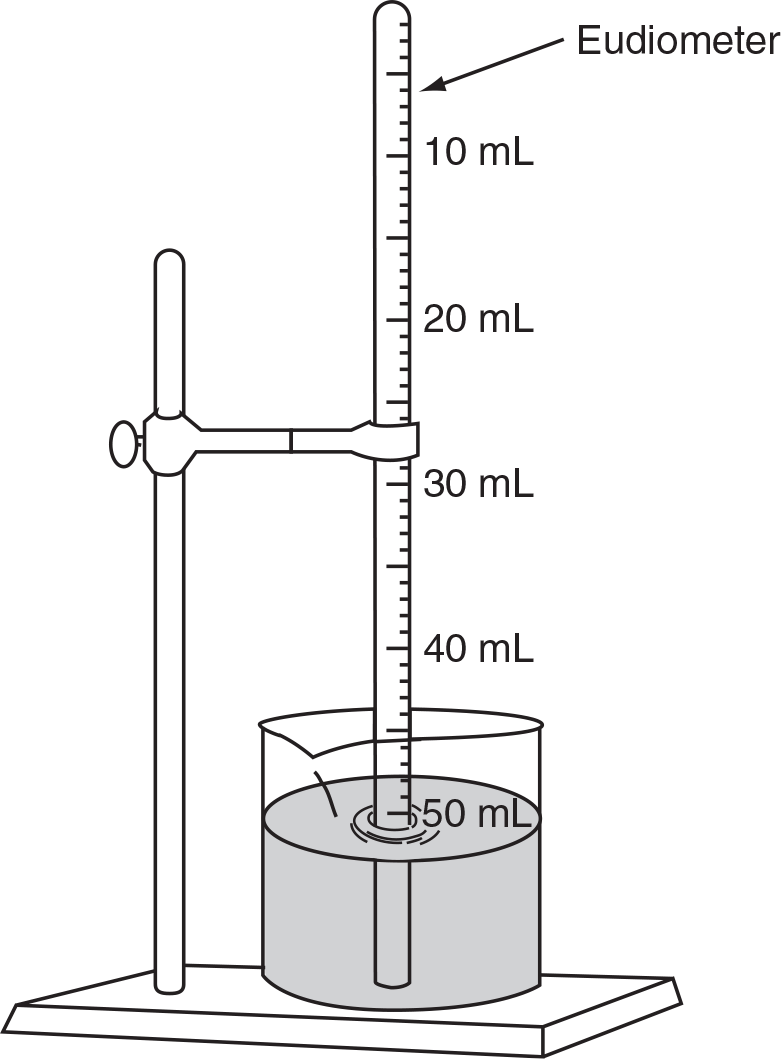
In this setup, a clean strip of magnesium with a mass of 0.040 g was introduced into the bottom of the tube, which contained a dilute solution of HCl, and allowed to react completely. The hydrogen gas formed was collected and the following data recorded:
Air pressure in the room = 730 mm Hg
Temperature of the water solution = 302 K
Vapor pressure of water at 302 K = 30.0 mm HgThe gas collected did not fill the eudiometer. The height of the meniscus above the level of the water was 40.8 mm.
-
What is the theoretical yield (in mL) at STP of hydrogen gas produced when the 0.040 g of Mg reacted completely?
- 10 mL
- 25 mL
- 37 mL
- 46 mL
- 51 mL
-
What is the correction to the atmospheric pressure due to the 40.8 mm height of the solution in the tube and above the level in the beaker?
- 3.0 mm Hg
- 6.0 mm Hg
- 13.6 mm Hg
- 27.2 mm Hg
- 40.8 mm Hg
-
What is the pressure of the collected gas once you have also corrected for the vapor pressure of the water?
- 730 mm Hg
- 727 mm Hg
- 30.0 mm Hg
- 697 mm Hg
- 760 mm Hg
-
Questions 12–14
- The rule is to add concentrated acid to water slowly.
- The rule is to add water to the concentrated acid slowly.
- Carefully replace unused or excess chemicals into their properly labeled containers from which they came.
- Flush eyes with water at the eyewash fountain for at least 15 minutes, and then report the accident for further help.
- Dispose of chemicals in the proper places and following posted procedures. Do not return them to their original containers.
-
Which of the above choices is the proper way to dilute a concentrated acid?
-
How do you properly dispose of chemicals not needed in the experiment?
-
What should you do if a chemical splatters into your eye?
-
What instrument is used in chemistry labs to measure the molarity of a colored solution by measuring the light transmitted through it?
- Electronic gravimetric balance
- pH meter
- Spectrophotometer
- Computer assisted probes
- Galvanometer