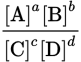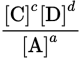Part A
Directions: Every set of the given lettered choices below refers to the numbered statements or formulas immediately following it. Choose the one lettered choice that best fits each statement or formula and record your answer on a separate sheet of paper. Each choice may be used once, more than once, or not at all in each set.
-
-
Questions 1–4 refer to the following diagram:

-
The activation energy of the forward reaction is shown by
-
The activation energy of the reverse reaction is shown by
-
The heat of the reaction for the forward reaction is shown by
-
The potential energy of the reactants is shown by
-
-
Questions 5–7 refer to the following diagram and information:
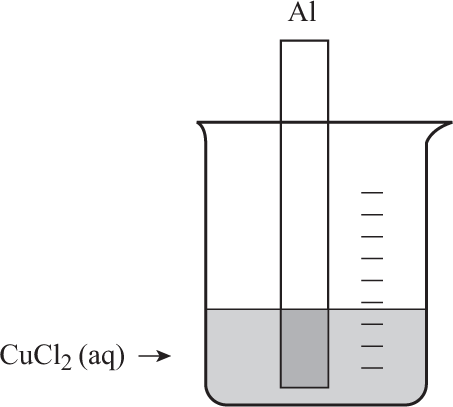
When a strip of aluminum is placed into a solution of copper(II) chloride, a reaction takes place. As time goes by, a brown solid forms on the strip of aluminum and the blue solution loses some of its color.
-
One product of the reaction would be
- AlCl2
- AlCl3
- AlCl4
- CuAl3
- CuAl2
-
The aluminum is
- being deprotonated
- being disproportionated
- being reduced
- being oxidized
- gaining mass
-
The copper(II) ion concentration in solution
- is zero at the beginning of the reaction
- has no effect on the rate of the reaction
- stays the same and doesn’t influence the color of the solution
- increases, making the solution less blue
- decreases, making the solution less blue
-
-
-
Questions 8–11 match the following equations to the appropriate descriptions:
- V/T = k
- P/T = k
- PV = k
- PT = P1 + P2 + P3 · · ·
- PT = k
-
This equation allows you to find the new pressure of a gas sample in a syringe when the plunger is pushed further into the barrel of the syringe when the temperature is held constant. It is an example of Boyle’s Law.
-
This equation allows you to find the new pressure of a gas in a rigid container when it is heated. It is an example of Gay-Lussac’s Law.
-
This equation allows you to find the new volume of a gas in a syringe when the syringe is placed into hot water and the atmosphere is impinging on the plunger with a constant pressure. It is an example of Charles’s Law.
-
This equation allows you to find the pressure of a mixture of carbon dioxide, methane, and nitrogen. It is an example of Dalton's Law.
-
-
-
Questions 12–14 refer to the following:
- 1
- 2
- 3
- 4
- 5
-
When the following reaction equation is balanced, what will be the coefficient of the NaNO3 using the smallest whole number?

-
If 0.5 moles of PbI2 formed according to the balanced equation from question 12, how many moles of NaI were needed to make it?
-
If NaNO3 goes into solution as ions, into how many ions would it dissociate?
-
-
-
Questions 15–18 refer to the following descriptions:
- Ionic substance
- Polar covalent substance
- Nonpolar covalent substance
- Amorphous substance
- Metallic network
-
MgCl2(s)
-
HCl(g)
-
CH3–CH3(g)
-
Cu(s)
-
-
-
Questions 19–23 refer to the following:
- Dissociation
- Amphoteric
- Phenolphthalein
- Dehydration
- Deliquescence
-
The reason why a blue crystal of CuSO4 · 5H2O turns white when heated
-
The reason why ionic substances dissolved in water exhibit conductivity
-
The reason why there may be a pink color in a basic solution
-
The reason why a substance may act like an acid or like a base depending on the substance it is in the presence of
-
The reason why an ionic solid may dissolve in the moisture it absorbs from the air
-
Part B
ON THE ACTUAL CHEMISTRY TEST, THE FOLLOWING TYPE OF QUESTION MUST BE ANSWERED ON A SPECIAL SECTION (LABELED “CHEMISTRY”) AT THE LOWER LEFT-HAND CORNER OF PAGE 2 OF YOUR ANSWER SHEET. THESE QUESTIONS WILL BE NUMBERED BEGINNING WITH 101 AND MUST BE ANSWERED ACCORDING TO THE FOLLOWING DIRECTIONS.
Directions: Every question below contains two statements, I in the left-hand column and II in the right-hand column. For each question, decide if statement I is true or false and if statement II is true or false and fill in the corresponding T or F ovals on your answer sheet. *Fill in oval CE only if statement II is a correct explanation of statement I.
For this practice test, record your answers on a separate sheet of paper.
Sample Answer Grid:
CHEMISTRY * Fill in oval CE only if II is a correct explanation of I.

-
I II Elements in the upper/left corner of the Periodic Table are active metals because metals have larger ionic radii than their atomic radii. -
I II A spontaneous reaction can be endothermic because an increase in the disorder of the system can outweigh the energy needs of a system and make a reaction spontaneous. -
I II Transition elements in a particular period may have the same oxidation number because they have a complete outer energy level. -
I II When a crystal is added to a supersaturated solution of itself, the crystal does not appear to change because the supersaturated solution is holding more solute than its normal solubility. -
I II Equilibrium is a static condition because at equilibrium, the forward reaction rate equals the reverse reaction rate. -
I II The ionic bond is the strongest bond because ionic bonds have electrostatic attraction due to the loss and gain of electron(s). -
I II In the equilibrium reaction N2(g) + 3H2(g) ↔ 2NH3(g) + heat, when the pressure in the reaction chamber is increased, the reaction shifts to the right because the increase in pressure causes the reaction to shift to the right to decrease the pressure since 4 volumes on the left become 2 volumes on the right. -
I II If the forward reaction of an equilibrium is exothermic, adding heat to the system favors the reverse reaction because additional heat causes a stress on the system, and the system moves in the direction that releases the stress. -
I II An element that has an electron configuration of 1s2 2s2 2p6 3s2 3p6 3d3 4s2 is a transition element because the transition elements from scandium to zinc are filling the 3d orbitals. -
I II The most electronegative elements in the periodic chart are found among nonmetals because electronegativity is a measure of the ability of an atom to draw valence electrons to itself. -
I II Basic anhydrides react in water to form bases because metallic oxides react with water to form solutions that have an excess of hydroxide ions. -
I II There are 3 moles of atoms in 18 grams of water because there are 6 × 1023 atoms in 1 mole. -
I II Benzene is a good electrolyte because a good electrolyte has charged ions that carry the electric current. -
I II Normal butyl alcohol and 2-butanol are isomers because isomers vary in the number of neutrons in the nucleus of the atom. -
I II The reaction of CaCO3 and HCl practically goes to completion because reactions that form a precipitate tend to go to completion. -
Part C
Directions: Every question or incomplete statement below is followed by five suggested answers or completions. Choose the one that is best in each case and then record your answer on a separate sheet of paper.
-
What are the simplest whole-number coefficients that balance this equation?

- 1, 6, 4, 2
- 2, 13, 8, 10
- 1, 6, 1, 5
- 3, 10, 16, 20
- 4, 26, 16, 20
-
How many atoms are present in the formula KAl(SO4)2?
- 7
- 9
- 11
- 12
- 13
-
All of the following are compounds EXCEPT
- copper sulfate
- carbon dioxide
- washing soda
- air
- lime
-
What volume of gas, in liters, would 2.0 moles of hydrogen occupy at STP?
- 11.2
- 22.4
- 33.6
- 44.8
- 67.2
-
What is the maximum number of electrons held in the d orbitals?
- 2
- 6
- 8
- 10
- 14
-
If an element has an atomic number of 11, it will combine most readily with an element that has an electron configuration of
- 1s2 2s2 2p6 3s2
- 1s2 2s2 2p6 3s2 3p1
- 1s2 2s2 2p6 3s2 3p2
- 1s2 2s2 2p6 3s2 3p5
- 1s2 2s2 2p6 3s2 3p6
-
An example of a physical property is
- rusting
- decaying
- souring
- low melting point
- high heat of formation
-
A gas at STP that contains 6.02 × 1023 atoms and forms diatomic molecules will occupy
- 1.06 qt
- 11.2 L
- 22.4 L
- 33.6 L
- 67.2 L
-
When excited electrons cascade to lower energy levels in an atom,
- visible light is always emitted
- the potential energy of the atom increases
- the electrons always fall back to the first energy level
- the electrons fall indiscriminately to all levels
- the electrons fall back to a lower unfilled energy level
-
Mass spectrometry uses the concept that
- charged particles are evenly deflected in a magnetic field
- charged particles are deflected in a magnetic field inversely to the mass of the particles
- particles of heavier mass are deflected in a magnetic field to a greater degree than lighter particles
- particles are evenly deflected in a magnetic field
-
The bond that describes an interaction between two orbitals that is not symmetrical about a line between the two atoms’ nuclei is called
- a pi bond
- a sigma bond
- a hydrogen bond
- a covalent bond
- an ionic bond
-
What is the boiling point of water at the top of Pikes Peak? (Note: Pikes Peak is well above sea level.)
- It is 100°C.
- It is >100°C since the pressure is less than at ground level.
- It is <100°C since the pressure is less than at ground level.
- It is >100°C since the pressure is greater than at ground level.
- It is <100°C since the pressure is greater than at ground level.
-
The atomic structure of the alkane series contains the hybrid orbitals designated as
- sp
- sp2
- sp3
- sp3d2
- sp4d3
-
Which of the following is (are) true for this reaction?

- It is an oxidation-reduction reaction.
- Copper is oxidized.
- The oxidation number of nitrogen goes from +5 to +4.
- I only
- III only
- I and II only
- II and III only
- I, II, and III
-
Which of the following properties can be attributed to water?
- It has a permanent dipole moment attributed to its molecular structure.
- It is a very good conductor of electricity.
- It has polar covalent bonds with hydrogen on opposite sides of the oxygen atom so that the molecule is linear.
- I only
- III only
- I and II only
- II and III only
- I, II, and III
-
All of the following statements are true for this reaction EXCEPT
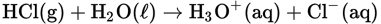
- H3O+ is the conjugate acid of H2O.
- Cl− is the conjugate base of HCl.
- H2O is behaving as a Brønsted-Lowry base.
- HCl is a weaker Brønsted-Lowry acid than H2O.
- The reaction essentially goes to completion.
-
What information about the subatomic particles in a particular carbon atom could be determined from the name carbon-13?
- The number of protons
- The number of neutrons
- The number of electrons
- I only
- III only
- I and II only
- I, II, and III
- II and III only
-
Which of the following salts will hydrolyze in water to form basic solutions?
- NaCl
- CuSO4
- K3PO4
- I only
- III only
- I and II only
- II and III only
- I, II, and III
-
When 1 mole of NaCl is dissolved in 1,000 grams of water, the boiling point of the water is changed more than when 1 mole of which other substance is added to the same mass of water?
- CaCl2
- C2H5OH
- AlBr3
- MgF2
- FeCl3
-
What is the structure associated with the BF3 molecule?
- Linear
- Trigonal planar
- Tetrahedron
- Trigonal pyramidal
- Bent or V-shaped
-
-
-
Questions 44 and 45 refer to the following setup:
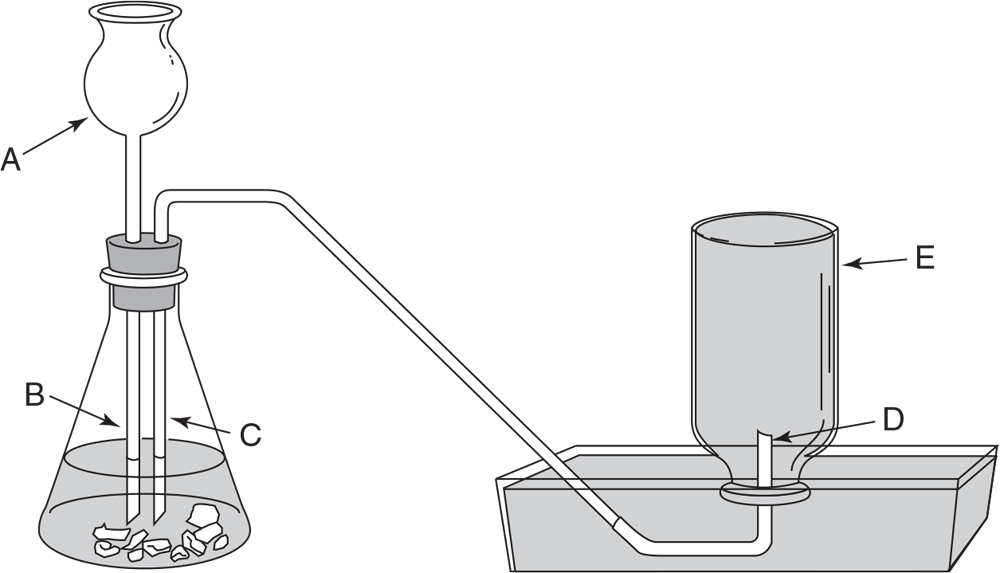
-
What letter designates an error in this laboratory setup?
- A (upper part of tube)
- B (lower part of tube)
- C
- D
- E
-
If the reaction in question 44 creates a gas, to where will the contents of the flask be expelled under these conditions?
- A
- B
- C
- D
- E
-
-
The most active nonmetal has
- a high electronegativity
- a low electronegativity
- a medium electronegativity
- large atomic radii
- a deliquescent property
-
In the reaction Fe + S → FeS, which is true?
- Fe + 2e− → Fe2+
- Fe → Fe2+ + 2e−
- Fe2+ → Fe + 2e−
- S → S2− + 2e−
- S2− + 2e− → S
-
What is the pH of a solution with a hydroxide ion concentration of 0.00001 mole/liter?
- −5
- −1
- 5
- 9
- 14
-
Hydrogen peroxide decomposes into water and oxygen according to the following equation:

The oxygen is being
- oxidized only
- reduced only
- both oxidized and reduced
- neither oxidized nor reduced
- acidified
-
· · · C2H4(g) + · · · O2(g) → · · · CO2(g) + · · · H2O(l)
If the equation for the above reaction is balanced with whole-number coefficients, what is the coefficient for oxygen gas?
- 1
- 2
- 3
- 4
- 5
-
It is found that 273 L of a gas at 0.821 atm and a temperature of 273 K has a mass of 40.0 grams. What is the molar mass of the gas in g/mol?
- 22.4
- 28.0
- 14.0
- 4.00
- 2.00
-
A compound whose molecular mass is 90.0 grams contains 40.0% carbon, 6.67% hydrogen, and 53.33% oxygen. What is the true formula of the compound?
- C2H2O4
- CH2O4
- C3H6O
- C3HO3
- C3H6O3
-
How many moles of CaO are needed to react with an excess of water to form 370 grams of calcium hydroxide?
- 1.0
- 2.0
- 3.0
- 4.0
- 5.0
-
To what volume, in milliliters, must 50.0 milliliters of 3.50 M H2SO4 be diluted in order to make 2.00 M H2SO4?
- 25.0
- 60.1
- 87.5
- 93.2
- 101
-
A small value of Keq indicates that equilibrium occurs
- at a low product concentration
- at a high product concentration
- after considerable time
- with the help of a catalyst
- with no forward reaction
-
A student measured 10.0 milliliters of an HCl solution into a beaker and titrated it with a standard NaOH solution that was 0.09 M. The initial NaOH burette reading was 34.7 milliliters while the final reading showed 54.7 milliliters.
What is the molarity of the HCl solution?
- 0.18
- 0.47
- 0.52
- 1.57
- 2.43
-
A student made the following observations in the laboratory:
- Sodium metal reacted vigorously with water, while a strip of magnesium did not seem to react at all.
- The magnesium strip reacted with dilute hydrochloric acid faster than did an iron strip.
- A copper rivet suspended in silver nitrate solution was covered with silver-colored stalactites in several days, and the resulting solution had a blue color.
- Iron filings dropped into the blue solution were coated with an orange color.
The order of decreasing strength as reducing agents is:
- Na, Mg, Fe, Ag, Cu
- Mg, Na, Fe, Cu, Ag
- Ag, Cu, Fe, Mg, Na
- Na, Fe, Mg, Cu, Ag
- Na, Mg, Fe, Cu, Ag
-
A sample of ethyl alcohol and water that were previously mixed in the laboratory must now be separated. Which separation technique could accomplish this?
- Filtration
- Magnetism
- Visual inspection
- Distillation
- Separating funnel
-
Which of these statements is NOT correct?
- In an exothermic reaction, ΔH is negative and the enthalpy decreases.
- In an endothermic reaction, ΔH is positive and the enthalpy increases.
- In a reaction where ΔG is negative, the forward reaction is spontaneous.
- In a reaction where ΔG is positive, ΔS may also be positive.
- In a reaction where ΔH is positive and ΔS is negative, the forward reaction is spontaneous.
-
A student filled a eudiometer with 32. milliliters of oxygen and 4.0 milliliters of hydrogen over mercury. How much of which gas would be left uncombined after the mixture was sparked?
- None of either
- 3.0 mL H2
- 24 mL O2
- 28 mL O2
- 30. mL O2
-
What would be the total volume, in milliliters, of gases in question 60 after sparking?
- 16
- 24
- 34
- 36
- 40
-
How can the addition of a catalyst affect an exothermic reaction?
- It speeds up the reaction.
- It slows down the reaction.
- It increases the amount of product formed.
- I only
- II only
- I and II only
- II and III only
- I, II, and III
-
In which period of the periodic table is the most electronegative element found?
- 1
- 2
- 3
- 4
- 5
-
Which expression represents the equilibrium constant for the reaction
 if A and D are solids?
if A and D are solids? -
-
Which of the following does NOT react with a dilute solution of sulfuric acid?
- NaNO3
- Na2S
- Na3PO4
- Na2CO3
- NaOH
-
Which of these statements is the best explanation for the sp3 hybridization of carbon’s electrons in methane, CH4?
- The new orbitals are one s orbital and three p orbitals.
- The s electron is promoted to the p orbitals.
- The s orbital is deformed into a p orbital.
- Four new and equivalent orbitals are formed.
- The s orbital electron loses energy to fall back into a partially filled p orbital.
-
The intermolecular force that is most significant in explaining the variation of the boiling point of water from the boiling points of similarly structured molecules is
- hydrogen bonding
- van der Waals forces
- covalent bonding
- ionic bonding
- coordinate covalent bonding
-
If K for the reaction
 is equal to 45.9 at 450°C and if both 1 mole of H2 and 1 mole of I2 are introduced into a 1-liter box at that temperature, what will be the expression for K at equilibrium?
is equal to 45.9 at 450°C and if both 1 mole of H2 and 1 mole of I2 are introduced into a 1-liter box at that temperature, what will be the expression for K at equilibrium? -
-
In the decomposition reaction of potassium chlorate, if 96.0 grams of oxygen are produced, the mass of potassium chloride produced would be
- 149.2 grams
- 74.2 grams
- 37.1 grams
- 96.0 grams
- unable to be determined
-
Alpha radiation
- bends away from atomic nuclei
- is the same as a stream of electrons
- is composed of hydrogen nuclei
- does not naturally occur
- has a neutral charge
-