Chapter 8
Manufacturing Protein
The genetic code dictates the sequence of amino acids in a protein molecule. The synthesis of proteins is quite complex, requiring three types of RNA. Messenger RNA (mRNA) contains the code and is the template for protein synthesis. Transfer RNAs (tRNAs) are adapter molecules that carry amino acids to the mRNA. Ribosomal RNAs (rRNAs) form part of the ribosome that brings together all the components necessary for protein synthesis. Several enzymes also help in the construction of new protein molecules. This chapter describes how the nucleotide sequence of an mRNA molecule is translated into the amino acid sequence of a protein.
Figure 8.1 shows the basic mechanism of protein synthesis, also called translation. In the first step, free amino acids are attached to tRNA molecules. In the second step, a ribosome assembles on the mRNA strand to initiate synthesis. In the third step, the ribosome travels along the mRNA. At each codon on the RNA a tRNA binds, bringing the amino acid defined by that codon to be added to the growing polypeptide chain. In the last, fourth, step the ribosome encounters a stop codon and protein synthesis is terminated.
Figure 8.1 Overview of protein synthesis.

Attachment of an Amino Acid to Its tRNA
Amino acids are not directly incorporated into protein on a messenger RNA template. An amino acid is carried to the mRNA chain by a tRNA molecule. tRNAs are small, about 7000 nucleotides in length, and are folded into precise three-dimensional structures because of hydrogen bonding between bases in particular stretches of the molecule. This gives rise to four double-stranded regions, and it is these that give tRNA its characteristic cloverleaf structure when drawn in two dimensions as in Figure 8.2.
Figure 8.2 Transfer RNA (tRNA).
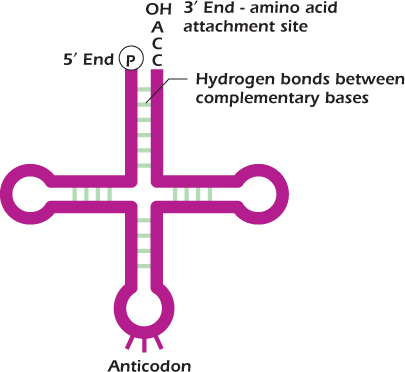
Each tRNA molecule has an amino acid attachment site at its 3′ end and an anticodon, three bases that are complementary in sequence to a codon on the mRNA. The tRNA binds to the mRNA molecule because hydrogen bonds form between the anticodon and codon. For example, the codon for methionine is 5′ AUG 3′ which will base pair with the anticodon 3′ UAC 5′.
Transfer RNA, the Anticodon, and the Wobble
Although 61 codons specify the 20 different amino acids, there are not 61 tRNAs; instead the cell economizes. The codons for some amino acids differ only in the third position of the codon. Figure 4.8 on shows that when an amino acid is encoded by only two different triplets the third bases will be either U and C, or A and G. For example aspartate is coded by GAU and GAC and glutamine by CAA and CAG. The wobble hypothesis suggests that the pairing of the first two bases in the codon and anticodon follows the standard rules—G bonds with C and A bonds with U—but the base pairing in the third position is not as restricted and can wobble. If the pyrimidine uracil (U) is in the third position of the codon, it can fit with any purine, G or A, in the 5′ position of the anticodon. Thus, only one tRNA molecule is required for two codon sequences. The anticodon of some tRNAs contains the unusual nucleoside inosine (I), whose base is the purine hypoxanthine (Fig. 2.13, . Inosine can base pair with any of U, C, or A in the third position of the codon. Some tRNA molecules can therefore base pair with as many as three different codons provided the first two bases of the codon are the same. For example, the tRNA for isoleucine has the anticodon UAI and can therefore base pair with any of AUU, AUC, or AUA.
The attachment of an amino acid to its correct tRNA molecule is illustrated in Figure 8.3. This process occurs in two stages, both catalyzed by the enzyme aminoacyl tRNA synthase. During the first reaction (A in the figure), the amino acid is joined, via its carboxyl group, to an adenosine monophosphate (AMP) and remains bound to the enzyme. All tRNA molecules have at their 3′ end the nucleotide sequence CCA. In the second reaction (B in the figure) aminoacyl tRNA synthase transfers the amino acid from AMP to the tRNA, forming an ester bond between its carboxyl group and either the 2′- or 3′-hydroxyl group of the ribose of the terminal adenosine (A) on the tRNA to form an aminoacyl tRNA. This step is often referred to as amino acid activation because the energy of the ester bond can be used in the formation of a lower energy peptide bond between two amino acids. A tRNA that is attached to an amino acid is known as a charged tRNA. There are at least 20 aminoacyl tRNA synthases, one for each amino acid and its specific tRNA.
Figure 8.3 Attachment of an amino acid to its tRNA.
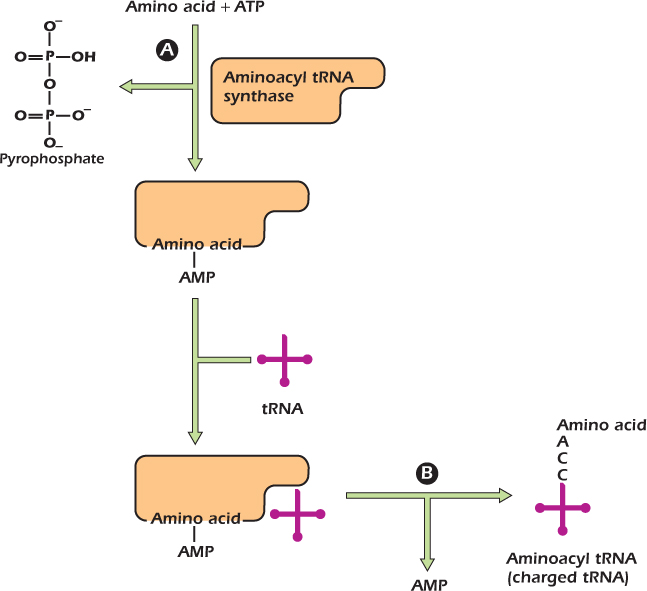
Figure 8.4 Prokaryote and eukaryote ribosomes.

The ribosome is the cell's factory for protein synthesis. Each ribosome consists of two subunits, one large and one small, each of which is made up of rRNA plus a large number of proteins. The ribosomal subunits and their RNAs are named using a parameter, called the S value. The S value, or Svedberg unit, is a sedimentation rate. It is a measure of how fast a molecule moves in a gravitational field. For example, the bigger a ribosomal subunit, the quicker it will sediment and the larger the S value. Prokaryotic ribosomes, and those found inside mitochondria and chloroplasts, are 70S when fully assembled and comprise a larger, 50S subunit and a smaller 30S one (Fig. 8.4A). Because the S value refers to the sedimentation rate (and not the molecular mass) of the ribosome or its subunits, 70S is less than the sum of 50S and 30S. Eukaryotic ribosomes are 80S when fully assembled and comprise a larger, 60S subunit and a smaller 40S one (Fig. 8.4B). The formation of a peptide bond between two amino acids takes place on the ribosome. The ribosome has binding sites for the mRNA template and for two charged tRNAs. An incoming tRNA with its linked amino acid occupies the aminoacyl site (A site), and the tRNA attached to the growing polypeptide chain occupies the peptidyl site (P site). The ribosome has a third binding site for tRNA, the exit site (E site). This is the site to which a tRNA moves before it leaves the ribosome.
IN DEPTH 8.1 HOW WE SEPARATE PROTEINS IN ONE DIMENSION
The technique known as SDS-PAGE is widely used to analyze the spectrum of proteins made by a particular tissue, cell type, or organelle. It is also invaluable for assessing the purity of isolated proteins. SDS stands for sodium dodecyl sulfate and PAGE for polyacrylamide gel electrophoresis.

The aim of the technique is to denature the proteins to be analyzed and then to separate them according to their size in an electrical field. To do this we first add the chemical 2-mercaptoethanol to the protein sample. This will break any disulfide bonds within a protein or between protein subunits. Next SDS, which is an anionic detergent, is added and the protein sample boiled. SDS coats each protein chain with negative charge. Each individual polypeptide in the sample becomes covered with an overall net negative charge. This means that when subjected to polyacrylamide gel electrophoresis the SDS-coated proteins will separate according to their size because the smaller proteins move most quickly toward the positive electrode or anode.
When electrophoresis is complete, the proteins are stained by incubating the gel in a solution of Coomassie brilliant blue. Each protein band stains blue and is detectable by eye. However, if the amount of protein is very low, a more sensitive detection system is needed, such as a silver stain. Proteins of known molecular mass are also electrophoresed on the gel. By comparison with the standard proteins, the mass of an unknown protein can be determined.
If we want to follow the fate of a single protein in a complex mixture of proteins, we combine SDS-PAGE with a technique called western blotting. The name western blotting is, like northern blotting, a play on the name of Dr. Ed Southern who devised the technique of Southern blotting to analyze DNA (Table 7.2 on ).
The protein mixture is separated by SDS-PAGE. A nylon membrane is then placed up against the polyacrylamide gel and picks up the proteins, so that the pattern of protein spots on the original polyacrylamide gel is preserved on the nylon membrane. The nylon membrane is then incubated with an antibody specific for the protein of interest. This antibody, the primary antibody, will seek out and bind to its target protein on the nylon membrane. A second antibody is added that will bind to the primary antibody. To be able to detect the specific protein of interest on the membrane, the secondary antibody is attached to an enzyme. In the figure shown, the enzyme used was horseradish peroxidase. A substrate is added and is converted by the enzyme into a colored product. The protein of interest is seen as a colored band on the nylon membrane. The same enzyme-linked secondary antibody can be used in other laboratories or at other times for western blotting of many different proteins because the specificity is determined by the unlabeled primary antibody.
Part A of the figure shows the Coomassie brilliant-blue-stained pattern of proteins isolated from the endoplasmic reticulum of liver. The leftmost lane is from a phenobarbital-treated animal while the middle lane is from an untreated control animal. The dark bands indicate the presence of protein. The spectrum of proteins is very similar in the two samples, except that a band with a relative molecular mass (Mr) of about 52,000 is darker in the sample from the treated animal. This tells us that drug treatment has caused an increase in the production of a protein with this relative molecular mass. Western blotting (part B) using an antiCYP2B1 antibody confirms that the induced protein is the cytochrome P450 protein known as CYP2B1. The CYP2B1 gene is activated by phenobarbital to produce more CYP2B1 protein to metabolize and clear the drug from the body .
We already showed how northern blotting revealed that transcription of the CYP2B1 gene is increased after phenobarbital treatment (Fig. 7.15 on ). The western blot shown here demonstrates that, as expected, the amount of CYP2B1 protein is increased as well.
Sodium dodecyl sulfate (SDS) is a major constituent of hair shampoo, where it is usually called by its alternative name of sodium lauryl sulfate.
Ribosome-Binding Site
For protein synthesis to take place, a ribosome must first attach to the mRNA template. AUG is not only the start codon for protein synthesis; it is used to code for all the other methionines in the protein. How does the ribosome recognize the correct AUG at which to begin protein synthesis? All bacterial mRNAs have at their 5′ end a stretch of nucleotides called the untranslated (or leader) sequence. These nucleotides do not code for the protein but are nevertheless essential for the correct placing of the ribosome on the mRNA. A nucleotide sequence 5′ GGAGG 3′ (or similar) is usually found with its center about 8 to 13 nucleotides upstream of (5′ of) the AUG start codon (Fig. 8.5). This sequence is complementary to a short stretch of sequence, 3′ CCUCC 5′, found at the 3′ end of the rRNA molecule within the 30S ribosomal subunit. The mRNA and the rRNA interact by complementary base pairing to place the 30S ribosomal subunit in the correct position to start protein synthesis. The sequence on the mRNA molecule is called the ribosome-binding site. This is sometimes referred to as the Shine–Dalgarno sequence after the two scientists who found it.
Figure 8.5 The initial binding of the prokaryote 30S subunit to mRNA. N indicates “any nucleotide.”

Because the genetic code is read in triplets of three bases, there are three possible reading frames . The reading frame that is actually used by the cell is defined by the first AUG that the ribosome encounters downstream of the ribosome-binding site.
Chain Initiation
The first amino acid incorporated into a new bacterial polypeptide is always a modified methionine, formyl methionine (fmet) (Fig. 8.6). Methionine first attaches to a specific tRNA molecule, tRNAfmet, and is then modified by the addition of a formyl group that attaches to its amino group. tRNAfmet has the anticodon sequence 5′ CAU 3′ that binds to its complementary codon, the universal start codon AUG.
Figure 8.6 Formyl methionine.

The 70S Initiation Complex
The initiation phase of protein synthesis involves the formation of a complex between the ribosomal subunits, an mRNA template, and tRNAfmet. Three proteins called initiation factors (IF), IF1, IF2, and IF3, together with the nucleotide guanosine triphosphate (GTP), are needed to help the 70S initiation complex form. First, the initiation factors bind to the 30S subunit (Fig. 8.7A). IF1 and IF3 attach to the 30S subunit. IF1 binds near to the site on the 30S subunit that will become part of the A site of the ribosome. IF2 is an enzyme that breaks down GTP to GDP and inorganic phosphate (Pi). IF3 prevents the 50S subunit binding to the 30S subunit. This is necessary to allow tRNAfmet to bind to an mRNA and a 30S subunit and to form the 30S initiation complex (Fig. 8.7B). The 50S subunit can now bind to 30S subunit; this is accompanied by the release of IF3 (Fig. 8.7C). Lastly, IF1 and IF2 are released and the GTP in IF2 is hydrolyzed, losing its γ phosphate to become guanosine diphosphate. The ribosome is now complete (Fig. 8.7D), and the first tRNA and its amino acid are in place in the P site of the ribosome. A 70S initiation complex has been formed, and protein synthesis can begin. The ribosome is orientated so that it will move along the mRNA in the 5′ to 3′ direction, the direction in which the information encoded in the mRNA molecule is read.
Figure 8.7 Formation of the prokaryote 70S initiation complex.

Elongation of the Protein Chain in Bacteria
The synthesis of a protein begins when an aminoacyl tRNA enters the A site of the ribosome (Fig. 8.8). The identity of the incoming aminoacyl tRNA is determined by the codon on the mRNA. If, for example, the second codon is 5′ AAA 3′, then lysyl tRNALys, whose anticodon is 5′ UUU 3′, will occupy the A site. The P site has, of course, already been occupied by tRNAfmet during the formation of the initiation complex.
Figure 8.8 Elongation of the protein chain.

Example 8.1 The Irritating Formyl Methionine
White blood cells are strongly attracted by any peptide that begins with formyl methionine: they assume an amoeboid shape (Fig. 1.5 on and begin to crawl toward the source of the peptide. To a white blood cell, the presence of a peptide beginning with formyl methionine means that there is an infection nearby that needs to be fought. This is because the body's own proteins do not contain formyl methionine: only prokaryotes begin protein synthesis with this modified amino acid.
In mice, scent-sensitive nerve cells in a specialized region of the nose respond strongly to formyl methionine peptides. The hypothesis, as yet unproven, is that this allows the mice to smell the presence of bacteria and therefore avoid spoiled food.
Elongation of a polypeptide chain needs the help of proteins called elongation factors (EF). The aminoacyl tRNA cannot bind on its own to the A site. Instead the aminoacyl tRNA must first form a complex with EF-Tu and a molecule of guanosine triphosphate (GTP) (Fig. 8.8A). The aminoacyl tRNA is now able to enter the A site (Fig. 8.8B). The presence of EF-Tu is important because it both guides and helps to correctly place the aminoacyl tRNA in the A site. However, for the peptide bond to form between two amino acids, EF-Tu must first be released from the ribosome. This happens when GTP is hydrolysed to GDP plus an inorganic phosphate ion (Pi). Now that both the A and P sites are occupied, the enzyme peptidyl transferase catalyzes the formation of a peptide bond between the two amino acids (fmet and lys in this example) (Fig. 8.8C). The dipeptide is attached to the tRNA occupying the A site.
For the polypeptide chain to grow, the ribosome must move down the mRNA, this is known as translocation. For this to happen the protein EF-G, bound to a molecule of GTP, enters the A site (Fig. 8.8D). When the GTP molecule is hydrolysed to GDP and inorganic phosphate (Pi) the ribosome moves (Fig. 8.8E). This causes the tRNA in the P site, which is no longer attached to an amino acid, to move to the E site (Fig. 8.8E) from where it is released from the ribosome (Fig. 8.8F). The tRNA and attached peptide chain move to occupy the P site. The movement of the ribosome releases EF-G from the A site (Fig. 8.8F) which is once again available for an incoming aminoacyl tRNA. The process of peptide bond formation, followed by translocation, is repeated until the ribosome reaches a stop signal and protein synthesis terminates. Proteins are synthesized beginning at their amino or N terminus . The first amino acid hence has a free (although formylated) amino group. The last amino acid in the chain has a free carboxyl group and is known as the carboxyl or C terminus .
IN DEPTH 8.2 PEPTIDYL TRANSFERASE IS A RIBOZYME
Peptidyl transferase, which catalyses the formation of the peptide bond between two amino acids, is not a protein molecule. In Escherichia coli it is the rRNA molecule within the large ribosomal subunit that is responsible for peptide bond formation and this is therefore called a ribozyme. This came as a surprise to scientists who discovered that the large subunit of the ribosome had peptidyl transferase activity even when all its protein had been removed.
The Polyribosome
More than one polypeptide chain is synthesized from an mRNA molecule at any given time. Once a ribosome has begun translocating along the mRNA, the start AUG codon is free, and another ribosome can bind. A second 70S initiation complex forms. Once this ribosome has moved away, a third ribosome can attach to the start codon. This process is repeated until the mRNA is covered with ribosomes. Each of these spans about 80 nucleotides. The resultant structure, the polyribosome or polysome (Fig. 8.9), is visible under the electron microscope. This mechanism allows many protein molecules to be made at the same time on one mRNA.
Figure 8.9 The polyribosome.

Termination of Protein Synthesis
There are three codons, UAG, UAA, and UGA, that have no corresponding tRNA molecule. These are stop codons. Instead of interacting with tRNAs, the A site occupied by one of these codons is filled by proteins known as release factors (RF) (Fig. 8.10). In the presence of these factors the newly synthesized polypeptide chain is freed from the ribosome. RF1 causes polypeptide chain release from UAA and UAG, and RF2 terminates chains with UAA and UGA. The RF proteins mimic the structure of tRNA. Despite being made up of amino acids instead of nucleotides, RF1 and RF2 have a very similar three-dimensional structure to tRNA. When the A site is occupied by a release factor (Fig. 8.10A), the enzyme peptidyl transferase is unable to add an amino acid to the growing polypeptide chain and instead catalyzes the hydrolysis of the bond joining the polypeptide chain to the tRNA. The carboxyl (COOH) end of the protein is therefore freed from the tRNA (Fig. 8.10B), and the protein is released. The release factors themselves must be removed from the ribosome. A third protein, RF3, helps remove RF1 from the ribosome.
Medical Relevance 8.1 Reading through a STOP
We described earlier (Medical Relevance 4.2 on ) how two nonsense mutations are responsible for the majority of cases of Hurler/Scheie syndrome. Remarkably, some antibiotics originally used to block translation in prokaryotes, such as gentamycin, allow eukaryotic ribosomes to travel on through a STOP codon, introducing an amino acid as they do so. Even better, the amino acid introduced at UAG is glutamine (normally encoded by CAA or CAG) meaning that the protein generated in cells from patients with one of the commonest mutations is completely normal. To date there have been no clinical trials of these drugs in Hurler/Scheie patients but the science looks very promising.
Figure 8.10 Termination of protein synthesis.

The Ribosome Is Recycled
The end of protein synthesis sees the polypeptide and release factor proteins dissociate from the ribosome. At this stage the ribosome is still bound to the mRNA and the P site is still occupied by a tRNA. Bacterial cells contain a protein called ribosome recycling factor (RRF). RRF, like the release factors RF1 and RF2, mimics the structure of a tRNA and binds to the empty A site (Fig. 8.11). The RRF protein is then able to recruit the EF-G protein, the same protein used in ribosome translocation. In a similar way to that described above, EF-G helps remove the tRNA from the P site. To dissociate the two ribosomal subunits, the initiation factor protein IF3 binds to the 30S subunit. The 30S subunit has been prepared to begin the assembly of the 30S initiation complex and a new round of protein synthesis.
Figure 8.11 The ribosome is recycled.

Eukaryotic Protein Synthesis is a Little More Complex
Elongation of the polypeptide chain and the termination of protein synthesis in eukaryotes does not differ very much from that described for bacteria. The elongation factors and release factors found in eukaryotes have different names to those found in bacteria, but the equivalent proteins do a very similar job. However, the initiation of protein synthesis is more complex in eukaryotes. Their proteins always start with methionine instead of the formyl methionine used in bacterial protein synthesis. A special transfer RNA, 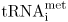 , is used to initiate protein synthesis from the AUG start codon. The methionine is often removed from the protein after synthesis. Eukaryotic mRNAs do not contain the bacterial Shine-Dalgarno sequence for ribosome binding. Eukaryotic mRNAs have at their 5′ end a 7-methyl guanosine cap . The cap is a key feature in the assembly of the ribosome on mRNA. The initiator
, is used to initiate protein synthesis from the AUG start codon. The methionine is often removed from the protein after synthesis. Eukaryotic mRNAs do not contain the bacterial Shine-Dalgarno sequence for ribosome binding. Eukaryotic mRNAs have at their 5′ end a 7-methyl guanosine cap . The cap is a key feature in the assembly of the ribosome on mRNA. The initiator 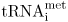 binds directly to the P site on the small ribosome subunit (the 40S subunit) along with several proteins called eukaryotic initiation factors (eIF). One of the eIF proteins binds directly to the cap and so positions the small subunit at the 5′ end of the mRNA. The small subunit then has to move forward to find the AUG start codon. It is helped to do this by other eIF proteins, which bind ATP that provides the power for the subunit to move. One of the eIFs is a helicase which unwraps any kinks in the mRNA produced by intramolecular hydrogen bonds so that the ribosome is able to slide along the mRNA. All eukaryotic mRNAs have a sequence very similar to 5′ CCACC 3′ adjacent to the initiating AUG codon. This sequence (known as the Kozak sequence after the scientist who noted it) tells the ribosome that it has reached the start AUG codon. The recognition of the AUG codon that specifies the start site for translation also requires the help of at least nine eIF proteins. Once the large 60S ribosomal subunit has attached to form the 80S initiation complex protein synthesis can begin.
binds directly to the P site on the small ribosome subunit (the 40S subunit) along with several proteins called eukaryotic initiation factors (eIF). One of the eIF proteins binds directly to the cap and so positions the small subunit at the 5′ end of the mRNA. The small subunit then has to move forward to find the AUG start codon. It is helped to do this by other eIF proteins, which bind ATP that provides the power for the subunit to move. One of the eIFs is a helicase which unwraps any kinks in the mRNA produced by intramolecular hydrogen bonds so that the ribosome is able to slide along the mRNA. All eukaryotic mRNAs have a sequence very similar to 5′ CCACC 3′ adjacent to the initiating AUG codon. This sequence (known as the Kozak sequence after the scientist who noted it) tells the ribosome that it has reached the start AUG codon. The recognition of the AUG codon that specifies the start site for translation also requires the help of at least nine eIF proteins. Once the large 60S ribosomal subunit has attached to form the 80S initiation complex protein synthesis can begin.
Example 8.2 The Diptheria Bacterium Inhibits Protein Synthesis
Some bacteria cause disease because they inhibit eukaryotic protein synthesis. Diphtheria was once a widespread and often fatal disease caused by infection with the bacterium Corynebacterium diphtheriae. This organism produces an enzyme (diphtheria toxin) that inactivates eukaryotic elongation factor 2 (the equivalent of the bacterial elongation factor G). Diphtheria toxin splits the bond between ribose and nicotinamide in NAD+ (Fig. 2.16 on , releasing free nicotinamide and attaching the remainder, ADP-ribose, to elongation factor 2, a process known as ADP ribosylation. The protein is now inactive and is unable to assist in the movement of the ribosome along the mRNA template. Protein synthesis therefore stops in the affected human cells. All the amino acids that the host was using to make its own protein are now available for the bacterium's use.
Antibiotics and Protein Synthesis
Many antibiotics work by blocking protein synthesis, a property that is extensively exploited in research and medicine. Many antibiotics only inhibit protein synthesis in bacteria and not in eukaryotes. They are therefore extremely useful in the treatment of infections because the invading bacteria will die but protein synthesis in the host organism remains unaffected. Examples are chloramphenicol, which blocks the peptidyl transferase reaction, and tetracycline, which inhibits the binding of an aminoacyl tRNA to the A site of the ribosome. Both of these antibiotics therefore block chain elongation. Streptomycin, on the other hand, inhibits the formation of the 70S initiation complex because it prevents tRNAfmet from binding to the P site of the ribosome.
Puromycin causes the premature release of polypeptide chains from the ribosome and acts on both bacterial and eukaryotic cells. This antibiotic has been widely used in the study of protein synthesis. Puromycin can occupy the A site of the ribosome because its structure resembles the 3′ end of an aminoacyl-tRNA (Fig. 8.12). However, puromycin does not bind to the mRNA. Puromycin blocks protein synthesis because peptidyl transferase uses it as a substrate and forms a peptide bond between the growing polypeptide and the antibiotic. Once translocation has occurred, the growing polypeptide has no strong attachment to the mRNA and is therefore released from the ribosome.
Figure 8.12 Puromycin can occupy the ribosome A site.

IN DEPTH 8.3 PROTEOMICS
Proteomics is the study of the proteome—the complete protein content of a cell. It is the proteins a cell makes that allow it to carry out its specialized functions. Although, for example, a liver and a kidney cell have many proteins in common, they also each possess a unique subset of proteins, which gives to them their own characteristics. Similarly, a cell will need to make different proteins, and proteins in different amounts, according to its metabolic state. The goal of proteomics is to identify all of the proteins produced by different cells and how a particular disease changes a cell's protein profile.

The separation of a cell's protein mixture into individual components is tackled using a technique known as two-dimensional polyacrylamide gel electrophoresis. This produces a pattern of protein spots. These patterns are recorded and serve as templates for the comparison of the proteomes of different cells. Spots that change during a cell's development, or changes that occur in disease, can be easily identified. A protein spot of interest is excised from the polyacrylamide gel and the protein broken into small, overlapping peptide fragments by proteolytic enzymes. The fragments are fed into a mass spectrometer, and their peptide mass fingerprint determined. The fingerprint identifies the protein.
The dream of many scientists, now that we know the base sequence of the human genome, is to define the human proteome and to determine all of its variations. However, the genome project used a single automated method to sequence the order of the four bases in DNA, and data was obtained at a relatively rapid pace. In contrast it is very labor intensive to identify proteins and much effort is being made to devise ways of speeding things up. The aim of proteomic research centers is to increase their throughput from about 40 to 100 peptide samples an hour, to the daunting number of 1 million peptides a day.
Protein Destruction
Different proteins have enormously different lifetimes. The keratin in hair lasts months until it is cut or falls off, while the α subunit of ATP synthase, the enzyme that makes ATP has a half life of 2 hours. Cytosolic proteins usually end their lives in the cell's equivalent of an office shredder, the proteasome. This is a barrel-shaped proteolytic machine that chops old or damaged proteins up into short lengths of peptide which are then in turn hydrolysed by cytosolic peptidases into their constituent amino acids, ready to be used again for protein synthesis.
1. Amino acids are prepared for use in protein synthesis by being attached to the 3′ end of a tRNA to form an aminoacyl tRNA.
2. The genetic code in an mRNA is translated into a sequence of amino acids on the ribosome, which comprises a small and large subunit and has two aminoacyl tRNA binding sites, the P site and the A site, plus an E site from which tRNAs are lost.
3. Initiation of protein synthesis involves the binding of the small ribosomal subunit to the mRNA. A special tRNA (tRNAfmet in prokaryotes, 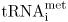 in eukaryotes) binds to the initiation codon, and then the large ribosomal subunit attaches and the initiation complex is formed.
in eukaryotes) binds to the initiation codon, and then the large ribosomal subunit attaches and the initiation complex is formed.
4. Protein synthesis begins when a second aminoacyl tRNA occupies the A site. Each incoming amino acid is specified by the codon on the mRNA. The anticodon on the tRNA hydrogen bonds to the codon, thus positioning the amino acid on the ribosome.
5. A peptide bond is formed, by peptidyl transferase, between the amino acids in the P and A sites. The newly synthesized peptide occupies the P site, and another amino acid is brought into the A site. This process of elongation requires a number of proteins (elongation factors); as it continues, the peptide chain grows.
6. When a stop codon is reached, the polypeptide chain is released with the help of proteins known as release factors.
7. More than one ribosome can attach to an mRNA. This forms a polyribosome, and many protein molecules can be made simultaneously from the same mRNA.
8. Many antibiotics fight disease because they inhibit particular steps in protein synthesis.
REVIEW QUESTIONS
The figure above shows a ribosome during translation to provide a reminder of the basic structure.

8.1 Theme: Translation Initiation
A. A site
B. E site
C. P site
D. 5′ AUG 3′
E. 5′ CCACC 3′
F. 5′ CCUCC 3′
G. 5′ CUG 3′
H. 5′ GGAGG 3′
I. 5′ UGCUUC 3′
J. Formyl methionine
K. Methionine
L. Poly-A tail
M. 7-methyl guanosine cap
From the above list of base sequences and other components, select the one relevant to each of the steps in translation initiation which are described below in sequence.
1. In the early stages of translation initiation in prokaryotes, the small ribosomal subunit attaches by complimentary base pairing to this sequence at the 5′ end of the mRNA, sometimes called the Shine-Dalgano sequence.
2. In contrast in the early stages of translation initiation in eukaryotes, the small ribosomal subunit attaches to this group at the extreme 5′ end of the mRNA molecule.
3. The eukaryotic small subunit then slides along the mRNA until it encounters this sequence, known as the Kozak sequence.
4. The subsequent steps are similar in prokaryotes and eukaryotes. The small subunit slides a few more bases along until it encounters this sequence, the start codon for translation.
5. Initiation factors then act to catalyze the assembly of the complete ribosome. The first tRNA, with its formyl methionine (in prokaryotes) or methionine (in eukaryotes) attached, locates in one of the three tRNA binding sites on the ribosome: state which one.
8.2 Theme: Translation Elongation and Termination
A. A site
B. E site
C. P site
D. EF-G
E. EF-Tu
F. IF-3
G. IF-Tu
H. puromycin
I. release factor 1 or 2
J. tRNAfmet in prokaryotes, 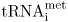 in eukaryotes
in eukaryotes
From the above list of proteins, compounds and components, select the one relevant to each of the steps in translation initiation which are described below in sequence.
1. Following translocation of the ribosome three bases along the mRNA, this site on the ribosome is empty and can be occupied by a charged tRNA whose anticodon is complimentary to the corresponding codon on the mRNA.
2. Peptidyl transferase now catalyzes the formation of a peptide bond between the new amino acid and the existing polypeptide chain. Immediately following this, the polypeptide chain is only attached to the mRNA via the tRNA in which of the three sites on the ribosome.
3. The next step is translocation, the physical movement of the ribosome three bases along the mRNA. Energy from this is provided by the hydrolysis of GTP by which enzyme, which occupies the A site on the ribosome.
4. As a result of translocation the uncharged tRNA, which gave up its amino acid during the formation of the peptide bond, moves to this site on the ribosome, from where it is released.
5. When translocation brings a stop codon UGA, UAA or UAG into the position facing the A site on the ribosome, the A site becomes occupied not by a normal charged tRNA molecule but rather by this molecule.
6. Finally the ribosome splits into two subunits as a result of energy released in GTP hydrolysis by EF-G. The released small ribosomal subunit already has one initiation factor attached, ready to accept the other initiation factors and the first charged amino acid to initiate synthesis of a new polypeptide. Name this pre-attached initiation factor.
8.3 Theme: The Wobble
You should refer to Figure 4.8 on while answering this question.
A. 5′ AAG 3′
B. 5′ CAU 3′
C. 5′ GAA 3′
D. 5′ GUU 3′
E. 5′ IAU 3′
F. 5′ UAI 3′
G. 5′ UUG 3′
H. 5′UAC 3′
From the above list of base sequences, select the one that is likely to be the anticodon on a tRNA specific for each of the amino acids below.
1. Methionine
2. Asparagine
3. Phenylalanine
4. Isoleucine
THOUGHT QUESTION
Predict the appearance of an SDS-PAGE gel used to study a purified sample of E. coli RNA polymerase .
Arnez, J. G., and Moras, D. (1997) Structural and functional considerations of the aminoacylation reaction. Trends Biochem. Sci. 22, 211–216.
Moore, P. B., and Steitz, T. A. (2002) The involvement of RNA in ribosome function. Nature 418, 229–235.
Ribas de Pouplana, L., and Schimmel, P. (2001) Aminoacyl-tRNA synthetases: Potential markers of genetic code development. Trends Biochem. Sci. 26, 591–596.
Shaw, K. (2008) The role of ribosomes in protein synthesis. Nature Education 1(1). www.nature.com/scitable/topicpage/The-Role-of-Ribosomes-in-Protein-Synthesis-1021.