Chapter 16
Intercellular Communication
The millions of cells that make up a multicellular organism can work together only because they continually exchange the chemical messages called transmitters. Here we describe how systems of cells use transmitters to cooperate for the good of the organism. Most of the many known transmitters are found in all animals, and probably evolved with ancestral multicellular organisms more than a billion years ago.
Classifying Transmitters And Receptors
Transmitter mechanisms can be classified in two ways. The first depends on the location and action of the receptor on the target cell. There are three types of receptors: ionotropic cell surface receptors, metabotropic cell surface receptors, and intracellular receptors.
Ionotropic Cell Surface Receptors
Ionotropic cell surface receptors are channels that open when a specific chemical binds to the extracellular face of the channel protein. The ionotropic glutamate receptor found on nerve cells (Fig. 16.1) is one example. The channel is closed in the absence of the amino acid glutamate in the extracellular medium. When glutamate binds, the channel opens and allows sodium and potassium ions to pass through. The electrochemical gradient pushing sodium ions into the cell is much greater than that pushing potassium ions out of the cell, so when the channel opens sodium ions pour in, carrying positive charge and depolarizing the plasma membrane. This mechanism is similar to that of two channels we met in Chapter 15, the inositol trisphosphate-gated calcium channel and the cAMP-gated channel. There is, however, a major difference: those two channels were opened by cytosolic solutes, while ionotropic cell surface receptors are opened by extracellular solutes.
Figure 16.1 The ionotropic glutamate receptor is an ion channel that opens when glutamate in the extracellular medium binds.

Metabotropic Cell Surface Receptors
Metabotropic cell surface receptors are linked to enzymes. We have already met a number of metabotropic cell surface receptors in Chapter 15. When the ADP receptor binds extracellular ADP, it activates Gq and hence phospholipase Cβ. The receptor for smell chemicals and the β-adrenergic receptor are linked to Gs and hence adenylate cyclase, so ligand binding increases cytosolic cAMP. Type 1 cytokine receptors are linked to JAK tyrosine kinase. Receptor tyrosine kinases are themselves protein kinases activated when their ligand binds.
The α-adrenergic receptor (Fig. 16.2) is another receptor that causes cytosolic calcium concentration to increase. The α- and β-adrenergic receptors are distinct proteins that bind the same transmitters, adrenaline and noradrenaline, but signal to different trimeric G proteins and therefore different downstream targets. To simplify the issue somewhat, we can say that noradrenaline acts mainly on α receptors and adrenaline acts mainly on β receptors. Because the α and β receptors are distinct proteins, it is possible to design drugs (α and β blockers) that interfere with one or the other.
Figure 16.2 Noradrenaline activates Gq and hence phospholipase Cβ in many cells including smooth muscle.

Example 16.1 A Toxic Glutamate Analog
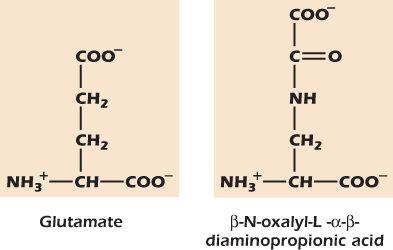
The grasspea is a protein-rich crop that has been cultivated since ancient times and is still an important source of calories and protein in India, Africa, and China. The peas contain the amino acid β-N-oxalyl-L-α − β-diaminopropionic acid. If untreated peas are eaten, the toxin binds to and opens the glutamate receptor on nerve cells. The resulting long-lasting depolarization damages and finally kills the nerve cells. Boiling the peas during cooking destroys the toxin; but in times of famine, when fuel is scarce, many people are poisoned, and the resulting brain damage is irreversible.
Intracellular Receptors
Intracellular receptors lie within the cell (in the cytosol or in the nucleus) and bind transmitters that diffuse through the plasma membrane. They always exert their effects by activating enzymes. The receptors for nitric oxide and steroid hormones are two examples.
Nitric oxide, or NO, is a transmitter in many tissues. It is not stored ready to be released but is made at the time it is needed. NO diffuses easily through the plasma membrane and binds to various cytosolic proteins that are NO receptors. One particularly important NO receptor is the enzyme guanylate cyclase, which in the presence of NO converts the nucleotide GTP to the intracellular messenger cyclic guanosine monophosphate, or cGMP.
Steroid hormones have intracellular receptors such as the glucocorticoid receptor . In the absence of hormone this receptor remains in the cytosol and is inactive because it is bound to an inhibitor protein. However, when the glucocorticoid hormone binds to its receptor, the inhibitor protein is displaced. The complex of the glucocorticoid receptor with its attached hormone now moves into the nucleus. Here two molecules of the complex bind to a 15-bp sequence known as the hormone response element (HRE), which lies upstream of the TATA box . The HRE is a transcriptional enhancer sequence. The binding of the glucocorticoid hormone receptor to the HRE stimulates transcription.
Classification by Transmitter Lifetime
The second way of classifying transmitter mechanisms depends on their lifetime in the extracellular medium. A transmitter that is rapidly broken down or taken up into cells acts only near its release site. One that is broken down slowly can diffuse a long way and may act on cells a long distance away. The shortest lived transmitters of all are those released at synapses , where the distance from release site to receptor is only 100 nm.
At the other extreme are transmitters that last many minutes or, sometimes, even longer. Hormones are long-lived transmitters that are released into the blood and travel around the body before being broken down. Most are released by specialized groups of secretory cells that form a structure called an endocrine gland. Paracrine transmitters also last many minutes before being broken down, but they are released into specific tissues rather than into the blood, and only diffuse within the tissue before they are destroyed.
Rapid Communication: From Nerve Cells To Their Targets
We have already introduced the synapse between the pain receptor and the pain relay nerve cell . An action potential in the axon terminal of the pain receptor raises cytosolic calcium from 100 nmol liter−1 to 1 μmol liter−1 and evokes release of the transmitter glutamate. Free glutamate survives only a few milliseconds in the extracellular medium and is quickly taken back up into cells by a carrier protein. However, its target—the pain relay cell—is only 100 nm away so it has time enough to bind to the ionotropic glutamate receptors present in the plasma membrane of the relay cell. These open, evoking a small depolarization (Fig. 16.3). However, the depolarization is not enough to take the transmembrane voltage to threshold which in these cells is about −40 mV. As soon as glutamate is removed from the extracellular medium, the transmembrane voltage returns to the resting level. In the axon of the relay cell, some distance from the synapse, the transmembrane voltage does not change, and no message passes on to the brain. The subject does not feel pain.
Figure 16.3 Opening of ionotropic glutamate receptors depolarizes the postsynaptic cell.

A subject does feel pain when they move their finger into the hot air from a hair dryer (Fig. 16.4). This is because the hot air heats a large area and causes many pain receptors to fire action potentials. Many of the pain receptors synapse onto one relay cell, which therefore receives many doses of glutamate. Because of this spatial summation, enough glutamate receptor channels open to depolarize the relay cell to threshold. Once threshold is reached the mechanism we described in Chapter 14, driven by sodium influx through voltage-gated sodium channels, takes over and an action potential travels rapidly up the axon of the relay cell to the brain so that the subject becomes aware of the painful event.
Figure 16.4 Spatial summation at a synapse.

An intense stimulus to a small area is also painful. When the subject is jabbed with a needle, only one pain receptor is activated, but that receptor is intensely stimulated and fires a rapid barrage of action potentials, each of which causes the release of glutamate and an extra depolarization of the pain relay cell (Fig. 16.5). Soon the transmembrane voltage of the relay cell reaches threshold, and an action potential travels along its axon toward the brain and the subject feels pain. Such temporal summation only occurs if the presynaptic action potentials are frequent enough to ensure that the depolarizations produced in the postsynaptic cell add.
Figure 16.5 Temporal summation at a synapse.

Inhibitory Transmission: Chloride-Permeable Ionotropic Receptors
The pain relay system is powerfully modulated by the brain. One component of this is mediated through the effects of another amino acid, γ-amino butyric acid (GABA) . Ionotropic GABA receptors are selective for chloride ions. Figure 16.6 illustrates a single nerve cell bearing both glutamate and GABA receptors.
Figure 16.6 Inhibition by a GABAergic synapse.

At point A on the graph an action potential in the GABA-secreting axon releases GABA onto the surface of the postsynaptic cell, causing the GABA receptor channels to open. Although chloride ions could now move into or out of the cell, they do not, because their tendency to travel into the cell down their concentration gradient is balanced by the repulsive effect of the negative voltage of the cytosol; chloride ions are at equilibrium . The opening of GABA channels therefore causes no ion movements and therefore does not alter the transmembrane voltage.
At point B, action potentials occur simultaneously in six glutamate-secreting axons. In this example, this activity provides enough glutamate to depolarize the postsynaptic cell to threshold, and the postsynaptic nerve cell fires an action potential.
At point C, action potentials occur simultaneously in six glutamate-secreting axons and also in the GABA-secreting axon. The same number of glutamate receptor channels open as before, and about the same number of sodium ions flow into the postsynaptic nerve cell, depolarizing it. However, as soon as the transmembrane voltage of the postsynaptic cell deviates from the resting value, chloride ions start to enter through the GABA receptor channels because the cytosol is no longer negative enough to prevent them from entering the cell down their concentration gradient. The inward movement of negatively charged chloride ions neutralizes some of the positive charge carried in by sodium ions moving in through the glutamate receptor channels. The postsynaptic nerve cell therefore does not depolarize as much and does not reach threshold. No action potential is generated in the postsynaptic nerve cell.
Glutamate and GABA are respectively the most important excitatory and inhibitory transmitters throughout the nervous system, and drugs that affect their operation have dramatic effects on neural processing. For example, antianxiety drugs such as Valium act on the GABA receptor and increase the chance of its channel opening. Nerve cells exposed to the drug are less likely to depolarize to threshold. Valium therefore reduces action potential activity in the brain, calming the patient.
How Nerve Cells Control the Body
We can use the gastrocnemius muscle to illustrate many of the concepts in this chapter (Fig. 16.7). This is the calf muscle at the back of the lower leg. When it contracts, it pulls on the Achilles tendon so that the toes push down on the ground. Most of the bulk of the muscle is made up of one type of cell, skeletal muscle cells. The plasma membrane of the skeletal muscle cell contains voltage-gated sodium channels, so like neurons they can generate action potentials.
Figure 16.7 Motoneurons release the transmitter acetylcholine that binds to nicotinic receptors on skeletal muscle cells. The plasma membrane of the muscle cell is depolarized to threshold and fires an action potential.

All skeletal muscle cells are relaxed until they receive a command to contract from nerve cells called motoneurons. Motoneuron cell bodies are in the spinal cord while the myelinated axons run to the muscle. To press the foot down, action potentials travel from the spinal cord down the motoneuron axon to its terminal, opening voltage-gated calcium channels in the plasma membrane, and transmitter is released into the synaptic cleft. Motoneurons are relatively unusual in using the chemical acetylcholine as their transmitter. The plasma membrane of the skeletal muscle cell contains ionotropic receptors for acetylcholine called nicotinic acetylcholine receptors, so named because the drug nicotine binds to them. Like ionotropic glutamate receptors, nicotinic acetylcholine receptors allow both sodium and potassium ions to pass, and cause a depolarization in the skeletal muscle cell. Indeed, just one action potential in a motoneuron elicits a depolarization so large that it depolarizes the skeletal muscle cell to threshold, which is about −50 mV in these cells. The resulting action potential in the muscle cell in turn causes release of calcium from the endoplasmic reticulum, and the increase of calcium concentration in the cytosol of the muscle cell causes it to contract .
Paracrine Transmitters And The Control Of Muscle Blood Supply
While transmission between nerve cells, and between nerve cells and skeletal muscle cells, is extremely rapid, communication between the various cells within a tissue takes seconds and minutes rather than milliseconds, and operates through paracrine transmitters. We can illustrate this by considering the control of blood flow to the muscle (Fig. 16.8). The inner surface of the blood vessel is a thin layer of epithelium called endothelium. Wrapped around these are muscle cells of a different type: smooth muscle cells. Both endothelial cells and smooth muscle cells are much smaller than skeletal muscle cells. A small blood vessel may be only as large as a single skeletal muscle cell. The flow of blood is controlled by the relaxation and contraction of the smooth muscle cells, which in turn is regulated both by local factors and by signals originating from outside the tissue.
Figure 16.8 Transmitters regulate the blood supply to muscles.

Exercise increases the blood flow to the muscles. A number of local mechanisms operate to cause this, including a simple action of deoxygenated hemoglobin in generating the transmitter nitric oxide. Blood contains up to 1 μmole liter−1 of the nitrite ion 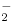 . When tissues take oxygen from hemoglobin, the now deoxygenated hemoglobin can reduce nitrite to nitric oxide:
. When tissues take oxygen from hemoglobin, the now deoxygenated hemoglobin can reduce nitrite to nitric oxide:
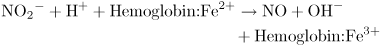
The nitric oxide easily passes through the plasma membranes of both endothelial and smooth muscle cells and reaches its receptor within the smooth muscle cells. Here it activates guanylate cyclase, causing an increase of cGMP concentration. (Meanwhile, as described in Medical Relevance 13.3 on , the Fe3+ in the hemoglobin must be reduced back to Fe2+ to restore hemoglobin function.)
Just as cAMP exerts many of its effects through cAMP-dependent protein kinase, called protein kinase A for short, so cGMP exerts many of its effects through another serine-threonine kinase called cGMP-dependent protein kinase or protein kinase G. One of the targets of protein kinase G is calcium ATPase . When this is phosphorylated by protein kinase G it works harder, reducing the concentration of calcium ions in the cytosol. This has the effect of relaxing the smooth muscle cells. The blood vessel therefore dilates, delivering more oxygen to the active tissue. Nitric oxide lasts for only about 4 seconds before being broken down. It is therefore a paracrine transmitter, able to diffuse through and relax all the smooth muscle cells of the blood vessel, but without lasting long enough to pass into more remote tissues.
Nitric oxide plays an important role in preventing turbulent flow in blood vessels all over the body. Physical stress of the plasma membranes of endothelial cells opens stretch activated channels that allow calcium ions to flow into the cells. Inside the endothelial cells is the enzyme NO synthase that makes nitric oxide, and this enzyme is activated by calcium. The nitric oxide then diffuses to the smooth muscle cells and relaxes them. The blood vessel therefore dilates, allowing a gentler flow rate to carry the same overall amount of blood.
Superimposed on these local mechanisms are controls from outside the tissue itself. The nervous system can act to divert blood from organs that are not in heavy use to areas that need it, such as active muscles. The most important nerve cells in this regard are those called vasoconstrictors because they cause blood vessels to contract. Action potentials in the axons of these cells cause the exocytosis of the transmitter noradrenaline onto the surface of the smooth muscle cells. The smooth muscle cells have α-adrenergic receptors in their plasma membranes. Binding of noradrenaline to α-adrenergic receptors activates PLCβ, which generates IP3, which in turn releases calcium from the endoplasmic reticulum into the cytosol. The increase of cytosolic calcium concentration causes the smooth muscle cells to contract, constricting the blood vessel and reducing the flow.
The Blood Supply Is Also Under Hormonal Control
The hormone adrenaline is chemically related to noradrenaline but is more stable, lasting a minute or so in the extracellular medium before being broken down. It is released from an endocrine gland (the adrenal gland) during times of stress and spreads around the body in the blood. Adrenaline that diffuses to the skeletal muscle cells stimulates them to begin breaking down glycogen to make glucose-6-phosphate . The smooth muscle cells of blood vessels within skeletal muscles also have β-adrenergic receptors connected to adenylate cyclase. However, they do not contain glycogen, and cAMP has another effect in these cells. When cAMP rises, it activates cAMP-dependent protein kinase. This in turn phosphorylates proteins that relax the smooth muscle cell. The action of adrenaline is therefore to increase the blood supply to all the muscles of the body in preparation for flight or fight. If we are very frightened and too much adrenaline is released, so much blood is diverted from the brain to the muscles that we faint.
New Blood Vessels in Growing Muscle
All the phenomena we have discussed so far occur within minutes. However, when a muscle is repeatedly exercised over many days, it becomes stronger: the individual skeletal muscle cells enlarge. This is because high cytosolic calcium acts via NFAT (Medical Relevance 10.1 on ) to stimulate the transcription of genes coding for structural proteins. Furthermore, new blood vessels sprout and grow into the enlarging muscle. A growth factor called FGF is released by stimulated muscle. The receptor for FGF, like that for PDGF , is found on endothelial and smooth muscle cells and like the PDGF receptor is a tyrosine kinase that signals via Ras and MAP kinase to trigger cell division and hence the growth of new blood vessels. (FGF stands for fibroblast growth factor, but FGF is effective on a vast range of cell types including both endothelial and smooth muscle cells).
In our study of the gastrocnemius muscle and its blood supply, we have seen examples of all types of transmitter mechanisms. Acetylcholine acts as a synaptic transmitter at the axon terminal of the motoneuron. Adrenaline is a hormone. The other transmitters are paracrine. The nicotinic acetylcholine receptor is an ionotropic cell surface receptor. The nitric oxide receptor is intracellular. The other receptors are metabotropic cell surface receptors. There is a wide variety of timescales of action. The acetylcholine released from the axon terminal of the motoneuron causes a contraction of the skeletal muscle cell within 5 milliseconds, by which time the acetylcholine has already been destroyed by extracellular enzymes. Adrenaline lasts 1 minute and dilates blood vessels for all this time. FGF lasts 10 minutes, but its effects are much longer lasting. FGF triggers the synthesis of proteins, which then act to cause cell proliferation that lasts for days. A similar pattern of intercellular communication, using some of the same transmitters and receptors but also many others, is found in every tissue of the body.
Motile cells are also affected by extracellular chemical cues, and one of their responses can be to orient their movement by the concentration gradient of the chemical. For example, peptides beginning with formyl methionine are a signal to the immune system that bacteria are close (Example 8.1 on . Neutrophils and other immune system cells are activated by N-formyl methionine peptides and crawl up the concentration gradient until they meet the bacterium, whereupon they will kill it and eat (phagocytose) it.
Following fertilization, a new embryo divides to generate a steadily increasing number of cells. These soon adopt particular traits so that as development proceeds choices made by cells lead them to adopt very specific identities such as motoneuron, skeletal muscle cell, or red blood cell. A focus of ongoing research and debate is the extent to which these decisions are reversible. With the single exception of the lymphocyte lineage described in Chapter 19, all the body's nucleated cells contain a complete genome and therefore could in principle generate the RNA and protein required to build any type of cell. Much research at present is aimed at persuading cells in the adult body to revert into more or less undifferentiated stem cells that can be used clinically to rebuild damaged tissue. Nevertheless, outside the laboratory the choices cells make are usually not reversed: neurons remain neurons, and muscle cells remain muscle cells. The cues that influence cell fate are of two types: those that are intrinsic to the cells themselves and those that arise elsewhere in the body.
Example 16.2 Viagra
Just as cAMP phosphodiesterase hydrolyzes cAMP to AMP and hence terminates its action as an intracellular messenger, so cGMP is inactivated by cGMP phosphodiesterase. There are a number of isoforms of this enzyme in different human tissues. The drug sildenafil, sold as Viagra, inhibits the form of the enzyme found in the penis. If cGMP is not being made, this has little effect on blood flow to the region. However, when cGMP is made in response to a local production of NO, its concentration in blood vessel smooth muscle increases much more than would otherwise occur because its hydrolysis to GMP is blocked. This in turn causes a greater activation of protein kinase G, a greater activation of the calcium ATPase, a lower cytosolic calcium concentration, a greater relaxation of blood vessel smooth muscle, and therefore greater blood flow.
Example 16.3 Nitroglycerine Relieves Angina
The discovery in 1987 that nitric oxide was a transmitter explained why nitroglycerine (more familiar as an explosive than as a medicine) relieved angina pectoris. Angina is a pain felt in an overworked heart. Nitroglycerine
spreads throughout the body via the bloodstream and slowly breaks down, releasing nitric oxide that then dilates blood vessels. The heart no longer has to work so hard to drive the blood around the body.
Intrinsic Cues
Intrinsic cues are in large part responsible for the ability of cells to retain their identity. For example, cells that make the decision to become muscle express the transcription factor MyoD, which then acts at the enhancer regions of a number of genes, including the gene for muscle myosin. MyoD also acts on the enhancer of the MyoD gene, causing more MyoD to be synthesized. This positive feedback ensures that once a cell has chosen to become a muscle cell, it remains one.
The best-characterized example of an intrinsic factor controlling a choice of cell fate is in the insect egg. The mRNA for the transcription factor bicoid is concentrated at one end of the ovoid egg cell (Fig. 16.9). After fertilization bicoid mRNA is translated, resulting in a spatial gradient of bicoid protein within the single cell. As the egg divides to form the multicellular early embryo, cells inherit different amounts of bicoid protein. Those that receive the most bicoid become, because of the action of bicoid at particular enhancer regions on DNA, the embryo's head. Cells receiving less and no bicoid become, respectively, mid-body and tail.
Figure 16.9 Bicoid signaling in the Drosophila embryo.

A similar mechanism operates during the development of the mammalian retina (Fig. 16.10). Within the stem cells of the retina of newborn rats the protein numb is found only along the surface that faces the pigment epithelium. If the stem cell divides symmetrically, so that both progeny cells inherit some of the numb, then both become photoreceptor cells. However if the stem cell divides asymmetrically, only the progeny cell that inherits the numb protein becomes a photoreceptor. The other becomes either a nerve cell or a glial cell. Figure 16.11 shows an image of dividing retinal stem cells.
Figure 16.10 Numb signaling in the vertebrate retina.

Figure 16.11 Cell division in the retina. The micrograph shows part of the developing retina. The tissue was fixed and then stained with propidium iodide, a dye that, like Hoechst, stains DNA, but fluoresces red. Four stem cells in the field of view have divided and are in telophase/cytokinesis, with the chromosomes still condensed and visible as independent structures . Three of the cell divisions, generating the daughter cells indicated by yellow arrows, were symmetrical divisions in the plane of the retina, but one, generating the daughter cells indicated by the green arrows, was an asymmetric one at 90 degrees to the plane of the retina. Image by Professor David Becker, University College London; used with permission.

Inductive Signaling
External signals are used throughout the body to allow one cell or group of cells to induce a specific direction of development in neighboring cells. In this chapter we have already seen an example in exercising muscle, where FGF released by active muscle triggers cell division in nearby endothelial and smooth muscle cells, leading to the creation of new blood vessels. The development of the motoneuron to skeletal muscle synapse yields a second example. During development the elongating axon of the motoneuron
secretes the protein agrin. When the axon gets close to a skeletal muscle cell, the agrin binds to and activates muscle-specific kinase, a receptor tyrosine kinase of the skeletal muscle cell. This then phosphorylates tyrosine residues within its own cytosolic domains, leading to the recruitment of proteins containing SH2 domains and, in turn, their phosphorylation. The end result is that nicotinic acetylcholine receptors are recruited to the site of active muscle specific kinases, creating the specialized postsynaptic zone.
Once synaptic transmission is set up, further changes are triggered. Depolarization of the muscle cell causes an increase of cytosolic calcium ion concentration that in turn activates the phosphatase calcineurin. Dephosphorylation of the transcription factor NFAT allows it to enter the nuclei of skeletal muscle cells and turn on the transcription of genes required for muscle cell growth and differentiation, such as muscle myosin.
1. Transmitter mechanisms can be classified in two ways. One depends on the location and action of the receptor on the target cell; the other on their lifetime and the extracellular medium in which the transmitter is found.
2. Receptors can be divided into ionotropic cell surface receptors, metabotropic cell surface receptors, and intracellular receptors. Ionotropic cell surface receptors are ion channels that open in response to ligand binding. The effect upon the target cell is electrical. Metabotropic cell surface receptors are linked to enzymes. The effect upon the target cell is mediated through a biochemical process. Intracellular receptors lie within the target cell and bind transmitters that are able to cross the plasma membrane by simple diffusion.
3. Synaptic transmitters are extremely short lived. Paracrine transmitters and hormones have a longer lifetime and are found in specific tissues and in the blood respectively.
4. Synapses between nerve cells do not generally mediate a one-to-one transmission of the action potential. The presynaptic signal must show summation in time or space to elicit a postsynaptic action potential.
5. Stimulation of ionotropic cell surface receptors that pass chloride ions makes it more difficult for a nerve cell to be depolarized to threshold.
6. The gastrocnemius muscle provides examples of all types of intercellular signaling operating in concert to fit the operation of the tissue to the requirements of the organism.
7. Gradients of extracellular chemical can cause chemotaxis, the directed movement of cells.
8. During development, the fate of cells can be determined by cues intrinsic to the cells themselves, such as the amount of a particular protein inherited from the progenitor cell, or by extrinsic cues such as transmitters released from neighboring cells.
REVIEW QUESTIONS
16.1 Theme: Receptors
A. α-adrenergic receptor
B. β-adrenergic receptor
C. glucocorticoid receptor
D. guanylate cyclase
E. interleukin 2 receptor
F. muscle-specific kinase
G. nicotinic acetylcholine receptor
From the above list of receptors, select the receptor that best fits each of the descriptions below.
1. A receptor that signals to the trimeric G protein Gq
2. A receptor that signals to the trimeric G protein Gs
3. A receptor tyrosine kinase
4. An intracellular receptor that is always cytosolic
5. An intracellular receptor that moves to the nucleus upon binding transmitter
6. An ionotropic receptor
16.2 Theme: Transmitters
A. adrenaline
B. GABA
C. glutamate
D. glycogen
E. lysine
F. noradrenaline
G. numb
H. Valium
From the above list of chemical species, select the chemical that best fits each of the descriptions below.
1. A hormone
2. A paracrine transmitter
3. A transmitter that acts to cause release of calcium from the endoplasmic reticulum of many cells including smooth muscle
4. A transmitter that is a γ-amino acid
5. A transmitter that is an α-amino acid
6. An excitatory synaptic transmitter
7. An inhibitory synaptic transmitter
16.3 Theme: Synapses
A. A depolarization that does not reach the threshold for action potential generation
B. A depolarization that exceeds the threshold for action potential generation
C. A long-lasting (more than one second in duration) switch to a membrane voltage more positive than 0 mV
D. A reduction in action potential frequency, or the complete cessation of action potentials
E. No voltage change
From the above list of electrical changes in postsynaptic cells, choose the change that would be evoked by each of the stimuli below.
1. A burst of activity in a presynaptic GABAergic neuron for a postsynaptic cell that is receiving steady excitatory input from a number of presynaptic glutaminergic neurons
2. A rapid burst of action potentials in a presynaptic pain receptor neuron caused by a painful stimulus such as a pinprick
3. A single action potential in a motoneuron
4. A single action potential in a presynaptic GABAergic neuron, at a time when no other synapses onto the postsynaptic cell are active
5. A single action potential in a presynaptic glutaminergic neuron, at a time when no other synapses onto the postsynaptic cell are active
THOUGHT QUESTION
In 1986 Hans Frohnhöfer and Christiane Nüsslein-Volhard reported an influential experiment in which a newly laid Drosophila egg was pricked at the anterior end and a drop of cytosol, representing about 5% of the total cell volume, squeezed out. The egg was allowed to develop into an embryo. What do you think Frohnhöfer and Nüsslein-Volhard observed?
Levitan, I. B., and Kaczmarek, L. K. (2002) The Neuron, 3rd edition, Oxford University Press, New York.
Marks, F., Klingsmüller, U., and Müller-Decker, K. (2009) Cellular Signal Processing. Garland, New York.
Wolpert, L., et al. (2007) Principles of Development, 3rd edition, Oxford University Press, Oxford.