
We saw in the last chapter that physics played an important role in many of the weapons of World War II, but it played an even larger role in the greatest weapon of the war—the atomic bomb. Indeed, it played a central role, for the atomic bomb is possible only because of our knowledge of fundamental physical concepts in physics. The subatomic particles that constitute the nucleus within the nucleus are bound together by what is called binding energy, and it is this binding energy that makes the atomic bomb possible.
The development of the atomic bomb is, without a doubt, one of the most impressive and awe-inspiring developments in history. Not it only did take a number of fundamental breakthroughs by a few ingenious thinkers, but it also took a tremendous effort by thousands of people to achieve it. And not only did these people achieve a goal that seemed almost impossible to many at first, but it showed what could be accomplished with enough motivation, determination, and ingenuity.
THE BEGINNING
It's hard to say exactly how it all began, but the experiments of James Chadwick of Cambridge University in England were critical. He was repeating an experiment done earlier by Irene Joliot-Curie and her husband Frederic Joliot-Curie in which a strange particle was able to knock protons out of paraffin. The Joliot-Curies thought the strange particle was a gamma ray. Chadwick showed that it was actually a neutrally charged particle, which he called a neutron.1 This neutral particle was in the nucleus of the atom along with the proton. As it turned out, the neutron had the same mass as the proton. The sum of the atomic weight of the protons and the atomic weight of the neutrons in an atom is approximately equal to the atomic weight (A) of the atom. We also designate the total number of protons in the nucleus as the atomic number (Z). And from these two numbers we can easily determine the number of neutrons in the nucleus; it is just A – Z. As an example, consider the hydrogen atom, which we know has a nucleus made up of a single proton. It has A = 1 and Z = 1, and since A – Z = 0, it has no neutrons. In the same way, the helium atom has A = 4 and two protons, or Z = 2, and since A – Z = 2, it has 2 neutrons. We can continue in his way through all the elements.
The neutron turned out to be a particularly important research particle because it was neutral. Early physicists tried to learn more about the nucleus by projecting high-speed particles at it to see what would happen. The only known particles available at the time, however, were the proton and the electron, but the electron was too light to have any effect on the nucleus, and the proton was positively charged, as was the nucleus, so the nucleus and the proton repelled one another. Because of this, the proton was also an ineffective projectile. The neutron, however, was not repelled electrically by the electrons or nucleus, so it was an ideal projectile. Before we look into how it was used, however, let's consider Einstein's contribution to the atomic bomb.
EINSTEIN'S ROLE
Einstein is sometimes called the father of the atomic bomb, a title he abhorred, and in reality he had very little to do with it directly. But he did make an important contribution. In a short paper published shortly after he published his famous paper on special relativity in 1905, he showed that energy and mass were related. The title of the paper was “Does the Inertia of a Body Depend on Its Energy Content?” It was only three pages long, but it was one of the most important papers ever published. This paper, along with a paper published a year later, showed an equivalence between mass and energy. In particular, it gave us the equation E = mc2, where the energy (E) of a given amount of mass is equal to the mass (m) multiplied by the speed of light (c) squared. The speed of light is 186,000 miles per second, and if you square it (multiply it by itself), you obviously get a very large number. This tells us that there is a large amount of energy associated with even a very small amount of mass. Fortunately, it is very difficult to transfer mass directly into energy, but this is, indeed, what happens in an atomic explosion.2
THE ITALIAN BREAKTHROUGH
Most physicists are either experimentalists or theoreticians. Enrico Fermi of the University of Rome, however, was one of very few people who excelled in both areas. He made important theoretical contributions, but at the same time he was a first-rate experimentalist. When the neutron was discovered in 1932, Fermi immediately realized that it would make an ideal projectile. It would not be repelled by the nucleus, and it could easily be projected fast enough so that the surrounding electrons would have no effect on it. The problem was finding a good source of neutrons, and he was soon able to devise an apparatus that would produce a beam of neutrons.3

Enrico Fermi.
One of the hottest areas in physics at the time was radioactive decay. A number of elements were known to spontaneously decay, emitting various types of radiation, referred to as alpha, beta, and gamma rays. A number of people, including Marie Curie, had made important contributions to the area. In 1934, however, Irene Curie and Frederic Joliot announced that they had been able to induce artificial radioactivity. In other words, they had caused a stable element to become radioactive. They had bombarded aluminum nuclei with alpha particles and caused it to become radioactive. They had also found that boron responded in the same way when bombarded with alpha rays.
Fermi was fascinated by the result, and he was sure he could improve on it.4 The alpha particles were big and heavy, and they could easily be stopped, even by a sheet of paper. Furthermore, they were charged. Neutrons would make a much better projectile, and he now had an apparatus for producing them. In addition, he had improved a device that had been invented several years earlier, called the Geiger Counter, which was used for measuring the radiation produced. Fermi and his group began by repeating the experiments of Joliot and Curie, and they quickly verified their results. They then turned to heavier elements. And indeed, many of them became radioactive, but in most cases the radioactivity was short-lived. Some of the elements, in fact, had half-lives (half-life is the time it takes for a mass of radioactive material to fall to half its original value) of less than a minute.5
Fermi and his team tested most of the elements of the periodic table in this way, all the way up to the heaviest known element at the time, namely uranium. And uranium was of particular interest to him. There were no known elements heavier than uranium, so he wondered what would happen if he shot a neutron at the uranium nucleus and it was absorbed. Would a new element be formed? Uranium has an atomic weight of 238 (total number of protons and neutrons in the nucleus); if it absorbed a neutron it should become uranium-239. But this created a new problem: how could they detect uranium-239? This proved to be frustratingly difficult. Finally, however, the team identified a slightly heavier element. Fermi was overjoyed. He had created an element beyond uranium-238. With this he concluded his experiments, but in doing so he failed to make one of the greatest discoveries in history.
In the meantime the world around him was becoming more and more turbulent. Hitler had seized power in Germany and Mussolini had signed a pact with him. Hitler's war against the Jews had already begun, and he demanded that Mussolini cooperate. Jews in Italy were therefore subjected to new legal restrictions. Fermi himself was not in danger, but Laura, his wife, was Jewish, and Fermi knew she might eventually be rounded up by authorities. He wasn't sure what to do. Government officials were unlikely to allow him to leave the country with his wife. Earlier he had been offered several positions at universities in the United States, but he had turned the offers down. He decided to write and ask if they were still interested, and indeed he got an offer from Columbia University. The problem now, however, was getting out of the country without arousing suspicion.
The breakthrough he was waiting for came in the fall of 1938. Fermi was in Copenhagen for a physics meeting when Niels Bohr took him aside and told him he was in line for the Nobel Prize that would be awarded later that year. Fermi was excited, not only at the prospect of winning the prize, but also because it might provide a possible route out of Italy. Indeed, a few weeks later he received a call informing him that he had won the prize, and that he would have to go to Stockholm, Sweden, to collect it. Furthermore, he was invited to take his family with him to Sweden. Immediately after the Nobel-Prize ceremony, Fermi boarded a plane for England, accompanied by his wife and children. From there they boarded a ship to New York.
HAHN, MEITNER, AND STRASSMANN
Lise Meitner was born into a Jewish family in Vienna, Austria, in 1878. She became interested in physics at an early age, but a scientific vocation was difficult for a woman at that time. Nevertheless, she managed to get a doctoral degree in physics at the University of Vienna. After obtaining it she went to the Kaiser Wilhelm Institute in Berlin and began working as an assistant to the chemist Otto Hahn.6 Initially she worked with no salary, but eventually she became head of a section in chemistry. She worked with Hahn for thirty years, making several important discoveries.7
When Hitler came to power in 1933 she was acting director of the Institute of Chemistry. Although she'd been born into a Jewish family, she had converted to Christianity early in her life, and as an adult she identified herself as Lutheran. Furthermore, she was Austrian by birth. So initially she wasn't worried by Hitler's action against Jews. She buried herself in her work. Others, including the Joliet-Curies in Paris had followed Fermi's lead in bombarding heavy elements, particularly uranium, with neutrons. Hahn and Meitner soon took an interest in their work.
Meitner had barely begun the work, however, when Hitler annexed Austria and issued a proclamation against all Jews, including those from Austria. Although she no longer considered herself a Jew, Meitner knew that that didn't matter to the Nazis. She had to get beyond the German area of influence as soon as possible, but there was a problem. Her visa had expired, and she could not apply for a new one because it would alert the authorities. She was uncertain about what to do, so she wrote Niels Bohr in Copenhagen. He made arrangements for her to get to Holland without a visa. But she still had to get past the Nazi patrols at the border. And, as she feared, a Nazi officer at the border asked her for her visa. She knew it had expired, but she handed it to him. He looked it over carefully as she sat in a state of fright. Finally, after several minutes he handed it back to her without saying anything. Minutes later, much to her relief, she was in Holland.
Bohr got her a position in Stockholm, Sweden, but it came with almost no support, and she was soon quite unhappy. Furthermore, Hahn and his assistant, Fritz Strassmann, were continuing the experiments they had started. Fermi had assumed that when uranium was bombarded with a neutron it would create a heavier, transuranic element, but he hadn't proved it beyond a doubt. But when Hahn and Strassmann did the experiment they were thoroughly confused. They couldn't verify Fermi's result; furthermore, an element, namely barium, with only about half the atomic weight of uranium, appeared to have been produced. It didn't make any sense, but Hahn had run the experiment through several times, getting the same result each time. Knowing that Meitner had a much better knowledge of nuclear physics than he did, he sent her a letter asking her if she had an explanation.
CHRISTMAS 1938
Meitner was amazed by the result, and confused. She had no explanation, but she was sure that Hahn had not made a mistake. If he said there was barium present after the bombardment, it had to be true. But where did it come from? Christmas neared, she pondered the strange result. She had a nephew, Otto Frisch, who was working for Bohr in Copenhagen, and she knew he was single. So she wrote to him to ask whether he would like to spend Christmas with her. He wrote back saying he would be delighted to spend it with her. He had been working on an interesting project related to the magnetic properties of the nucleus, and he was anxious to tell her about it, as she might have some suggestions for him.8
He was a little disappointed, however, when he met her. She immediately began talking about the letter she had received from Hahn. She finally handed it to him to read. Frisch suggested that the strange outcome of Hahn's experiment might be an error due to contamination, but she argued that Hahn was too good a chemist to allow that. They continued talking about it for some time.
In general, only small particles such as electrons, neutrons, and alpha particles were observed in nuclear reactions. A heavier or slightly lighter nucleus might be produced, but there seemed to be no way that a nucleus with half the atomic weight of uranium would be produced. The only way it could be produced is if the uranium nucleus had somehow broken in half. But that was impossible—the energy required for something like that had to be incredibly large, and the neutrons that hit the nucleus only had a small energy.
Frisch had brought his skis and wanted to do some cross-country skiing while he was there, so off they went, Frisch on skis and Meitner walking in the snow. They began talking about the two models of the nucleus. Ernest Rutherford had suggested it was a small, rigid ball, but Bohr had recently put forward a new and different model that was quite controversial at the time. He suggested that the nucleus was actually relatively soft and pliable—more like a drop of water.
There was no way Rutherford's model would allow a splitting into two nuclei, each half the size of the original one. Bohr's model, however, might work. They paused and sat down on a fallen tree near the path. Lise pulled out a piece of paper and a pencil from her pocket. She drew a picture of a uranium nucleus, assuming that it was a sphere. What would happen to it if a neutron hit it? If it was like a drop of water, it could change its shape slightly; it could become elongated. Meitner began to calculate the forces on the drop. Cohesive forces held the drop together, so if it eventually broke apart, these forces would have to be overcome. And the cohesive force was related to the surface tension of the drop. The force that might overcome it had to come from the charge of the nucleus. And indeed, the large, unstable uranium nucleus would likely wobble. If so, it would become elongated at first, but as it continued to oscillate it might begin to resemble a dumbbell, and if this happened, the two masses at the ends of the dumbbell would repel one another as a result of their similar charges.9
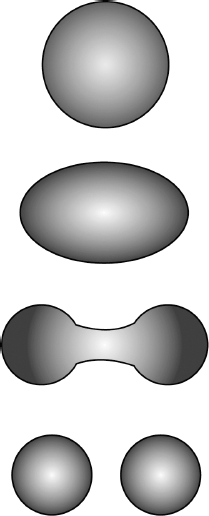
A wobbling drop that fissions into two smaller drops.
Meitner calculated how much energy would be released if this occurred. She was surprised to find that it would be about 200 million electron volts (an electron volt is the energy an electron gains in passing through a voltage difference of 1 volt). This was not a large amount, but when multiplied by the number of nuclei that would be splitting, it would be very large. But where did this energy come from? Meitner immediately thought about a lecture she had attended many years earlier at which Einstein had given a formula relating mass and energy. She added the masses of the two product nuclei and compared the sum with the mass of uranium. Then she used Einstein's formula to convert the difference in mass to energy. Amazingly, the result was the same: 200 million electron volts. This was obviously not a coincidence. Uranium nuclei had split in half—an amazing discovery if indeed that was what had happened. They decided to publish their results as soon as possible.
Frisch rushed back to Copenhagen. He could hardly wait to tell Bohr. But Bohr was getting ready for a trip to the United States and couldn't spend much time with him. Nevertheless, he was delighted with the news, and he encouraged Frisch and Meitner to publish as soon as possible. Frisch began writing up the paper, but he was stumped by the problem of how to describe the splitting. A friend noted that it was quite similar to the breaking apart of a simple cell in biology, and that was called “fission.” The name “nuclear fission” immediately came to mind, and he used the phrase in the article. It was published five weeks later in the scientific journal Nature.
By then Hahn had published his result, but Meitner had not yet told him of the interpretation that she and Frisch had developed, so there was no mention of fission in his paper. Meitner, in fact, hesitated for a while before she told Hahn. She wanted to be sure that the paper she and Frisch had written was published first. There was some irony in all of this, however. Hahn was awarded the Nobel Prize in 1944 for his discovery of fission, with no mention of Meitner, even though she was the one who interpreted his result as fission.
A CHAIN REACTION
Bohr could hardly contain his excitement about the new discovery as he sailed to America. Along with coworker Leon Rosen, he tried to work out the details of what might happen during the fission of a uranium nucleus. There was no doubt that a tremendous amount of energy would be released. Could it be used to make a bomb? The possibility worried him. He had promised Frisch that he wouldn't mention the discovery until after Frisch and Meitner had published their results, but he forgot to mention this to Rosen.
Bohr, Rosen, and their group were met in New York by Fermi, Fermi's wife, and John Wheeler, a former student of Bohr's. Bohr said nothing about the discovery, but within a short time he discovered that everyone seemed to know about it. Then he realized he had forgotten to tell Rosen to keep it secret. The secret was now out, so he decided to make an announcement at a Washington conference on theoretical physics that he would be attending within a few days. Many of the world's top physicists were at the meeting, including Hans Bethe, Edward Teller, George Gamow, Harold Urey, Isidor Isaac Rabi, Otto Stern, and Gregory Breit. As expected, everyone was stunned when the news was announced, particularly after Bohr mentioned that a super bomb might be possible using nuclear fission.
When Fermi heard about the discovery, he had mixed emotions; he realized he had come very close to making the discovery himself, and he was annoyed. But at the same time he realized it was a momentous discovery, and it was important to follow up on it as quickly as possible. He immediately set up a simple experiment at Columbia University to verify the result, and he was pleased to see that there was no doubt: uranium nuclei did, indeed, fission.
Everyone was talking about the new discovery, and as Bohr, Wheeler, Fermi, and Leo Szilard got together for dinner the day after the conference they were still tossing around ideas about it. One of the most interesting of these ideas was one that Bohr had casually mentioned at the meeting. He pointed out that if the uranium nucleus split in half, leaving two lighter nuclei, there would have to be some neutrons left over, but he wasn't sure how many. Nevertheless, if there were two or more neutrons coming out of the reaction, each of them might produce a new fission. Furthermore, it was like the old story of the employer offering a new employee a wage of one cent the first day, then doubling the wage each day thereafter. After rejecting it, the employee realizes that he would have been a millionaire within a month. In essence, it doesn't take much doubling before small numbers become huge. And since each of the new fissions would take place in a tiny fraction of a second, an incredible amount of energy would be released very rapidly.10
The possibility was so exciting that Bohr asked Wheeler if he would like to work in collaboration with him to see what was possible, and Wheeler agreed. But they soon found that they would need some additional experimental results, so an experiment was set up at Princeton University to find out how the rate of fission would be affected by the speed or energy of the incoming, or bombarding, neutrons. In particular, they wanted to find out if there was a significant difference between slow and fast neutrons. They began bombarding uranium with extremely energetic neutrons, and, as expected, the higher the energy of the neutron, the greater the fission rate. But they also got an unexpected result: at very low neutron energies, the rate of fission also increased. In essence, the fission rate was high for slow neutrons and also for very fast neutrons. This seemed a little crazy. Bohr and Wheeler thought about it. The reason for this had to be related to the uranium they were using, which was natural uranium that had come out of the ground.
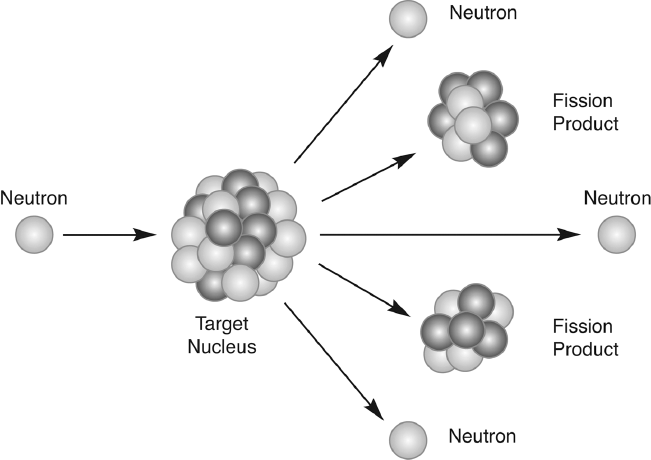
Fission creating a chain reaction.
To understand why this is important we have to go back to the elements and look more closely at how they are made up. As we saw earlier, they have a certain number of protons and neutrons in their nucleus (we will ignore the electrons because they are irrelevant for this discussion). Furthermore, each element is identified by a mass number (A) (closely related to the atomic weight), and an atomic number (Z). The mass number is equal to the number of protons plus the number of neutrons, while Z is the number of protons in the nucleus, and it's the number of protons in the nucleus that uniquely defines an element. For example, the carbon nucleus has six protons, but it can also have either seven or eight neutrons. This difference in the number of neutrons does not change it into a new element; rather, the different numbers of neutrons identify different isotopes of the same element. And, as it turns out, uranium also has two isotopes that differ in the number of neutrons they contain. They are referred to as U-238 and U-235. Natural uranium is a mixture of these two isotopes.
As Bohr and Wheeler looked closely at the results of the Princeton experiment they realized that the sudden increase in fission as a result of the bombardment with slow neutrons was due to U-235. The increase with fast neutrons was mainly due to U-238. This meant that U-235 fission required less energy than U-238 fission. Thus, U-235 would be much better for a bomb, particularly because secondary neutrons were very slow. The problem was that natural uranium consisted almost entirely of U-238; only 0.7 percent of natural uranium was U-235. And to make things even worse, because they were chemically the same, there was no chemical process that could separate U-235 from U-238. Some sort of physical process, such as diffusion, would have to be used, and it would be difficult to do.
Although they didn't realize it at the time, others were already thinking along the same line. Irene Joliet-Curie and her husband Frederic, in Paris, also realized that a bomb might be possible. Furthermore, Otto Hahn, who was still in Nazi-controlled Germany, would no doubt soon come to the same conclusion. In addition, Werner Heisenberg, one of the brightest and most famous physicists in the world, was also in Germany, along with several other world-renowned physicists. One who was particularly worried about the bomb-making implication of nuclear fission was Leo Szilard.
THE LETTER TO ROOSEVELT
Leo Szilard had come to America several years earlier from Germany. He was Jewish, and when Hitler came to power he knew his days in Germany were numbered. In 1933 he went to England, and later moved on to the United States. Interestingly, about this time he had already begun to think about the possibility of a super bomb. He told Fermi about his worry, but Fermi didn't take him seriously; at this stage Fermi was not yet convinced that a bomb could be built. Disappointed in Fermi's response, Szilard decided to do something about it on his own.11 He knew that one of the largest known deposits of uranium in the world was in the Belgian Congo. And as soon as German scientists realized how important uranium was, they would rush to buy up as much of it as possible. Szilard had to stop them. He remembered that Einstein was a personal friend of Belgium's queen. He immediately phoned Einstein, who was now at Princeton's Institute for Advanced Study, but he was told that Einstein was at his summer home on Long Island.
Szilard acquired Einstein's address, but he now had a problem. He had never learned to drive a car, so he had to get his friend Isidor Isaac Rabi to drive him. After some trouble, they finally found their destination and were greeted by Einstein. Szilard told him the news, and Einstein was surprised; he had heard nothing about the new discoveries, but he was immediately concerned. He knew that if the Germans produced such a bomb they would likely use it, and it worried him. Szilard told him about the uranium deposits in the Belgian Congo and suggested that he write a letter to Elizabeth, the queen of Belgium. Einstein was reluctant to bother her, but he offered to write a letter to a friend who was in the Belgian cabinet.
They talked about other things they could do. A letter to the White House was suggested. Szilard knew, however, that a letter signed by him would be ignored whereas a letter from Einstein would be taken seriously. Einstein agreed to sign a letter written by Szilard. The next problem was getting the letter to Roosevelt; it had to be delivered to him directly to have any impact. Szilard remembered an acquaintance by the name of Alexander Sachs, who sometimes visited Roosevelt. Szilard gave him the letter on August 15, 1939, and Sachs agreed to deliver it.
Germany was on the verge of attacking Poland at this time, however, and Roosevelt was particularly busy. Sachs tried several times but was unable get an appointment. He finally succeeded in October 1939. Roosevelt agreed that action was needed, and he authorized the creation of an advisory committee on uranium. The committee had its first meeting on October 21, at which six thousand dollars was budgeted for conducting experiments on neutrons. Szilard was disappointed with the small amount of money, but at least it was a first step.
Several problems would have to be overcome, however, before they could build a bomb. First of all, the uranium would have to be purified. At that time uranium had no known uses and very little of it had been mined, and the small amount that had been produced was not pure. Furthermore, Bohr and Fermi had showed that it was U-235 that was of most interest, and there was only a small amount of it in natural uranium. The U-235 would have to be separated out. And perhaps of most interest, some sort of device would have to be built to slow down the fission process so that it could be controlled. Such a device would be needed in order to determine whether a bomb would be possible. This device was eventually called a nuclear reactor. And for a controlled reaction, a moderator would be needed (a material that would absorb some of the neutrons coming out of the reaction). Two moderators were known: heavy water and graphite. Heavy water was expensive, so graphite seemed to be the better choice.
THE WAR BEGINS
In September 1939 Germany invaded Poland and World War II began. Because of Hitler's policies regarding Jews, many Jews had fled Germany, including Albert Einstein and other top physicists. But Werner Heisenberg, who had just won a Nobel Prize, was not Jewish, and he had no interest in leaving. He had been offered positions at several major universities in the United States, but he had turned them down. As a German, he felt compelled to serve his country in its time of need. And indeed, by the time the war started, the Nazi government had also heard about the possibility of a super bomb. In fact, a group of Germany's leading scientist (the few that were left) had been recruited to study uranium fission. The group was referred to as the Uranverein (Uranium Society), and one of the most prominent members was Otto Hahn, the man who had discovered fission.12 Members of the Uranverein were reluctant at first to invite Heisenberg because he was a theorist, and the building of a bomb needed experimentalists. Furthermore, he had been friends with many Jewish scientists, including Einstein, so he was not considered desirable. Interestingly, Hahn was a rather reluctant member of the group, and in the end he made almost no contribution to it. He continued to raise objections against the project, sure that it would never be possible. The Uranverein finally decided to invite Heisenberg into the group in late September 1939 to help solve what seemed to be insurmountable problems. Soon he was leader of the group.
Heisenberg realized that the first step had to be the building of a nuclear reactor: a slowed-down bomb. And the best moderator was heavy water, or deuterium. But deuterium was not plentiful in Germany; however, it was being produced in a plant in Vemork, Norway. Germany had not yet invaded Norway, so would have to purchase the deuterium it needed. The Vemork plant was perched high above the fjords in a remote region of Norway, about 150 miles from Oslo.
The Germans approached the owner of the Vemork plant and offered to buy all its available heavy water. The Norwegians were surprised by the offer and wondered why the Germans would need so much. When they were not given an answer they refused to sell. About the same time, the Joliet-Curies in Paris had arrived at the same conclusion as the Americans and Germans. For a bomb, a reactor would be needed, and it would need heavy water as a moderator. The French government therefore also sent a representative to Vemork, and when he told Norwegian officials what the heavy water was needed for they promised him all they had at no cost.
Then, in April 1940, Germany invaded Norway, changing the situation. The German army raided the plant immediately and was disappointed to find that all the heavy water had been shipped to France. So the Germans immediately ordered an acceleration in production and insisted that everything that was produced was to be shipped to Berlin.
In early June 1940, Germany also invaded France, and when Paris fell on June 14, the Uranverein physicists immediately went to the Joliet-Curie laboratory, expecting to find heavy water and uranium. Joliet-Curie claimed that it had been loaded onto a ship that had been sunk, and the Germans accepted the answer. The materials had actually been shipped to England.
By now the Germans had made considerable progress. One of the Uranverein group had calculated that uranium would have to be enriched so that it contained 70 percent more U-235 than U-238 before it could be used in a reaction. They had also discovered that when U-238 captured a neutron it formed U-239, which was unstable and radioactively decayed in twenty-three minutes. The new element, which was unnamed, might be fissionable according to Carl Friedrich von Weizsäcker, one of the group members. This meant that if they could build a reactor they might have a way of producing another element that could be used for a bomb.
In the summer of 1940 a new building at the Kaiser Wilhelm Institute in Berlin was designated for the exclusive use of the Uranverein. It was next door to the Institute of Physics and was called the Virus House. Germany now had a good supply of uranium, ample heavy water, and several important developments from the Joliot-Curie lab. But the members of the Uranverein were soon stumped by the difficulty of separating U-235 from natural uranium.
MEANWHILE IN ENGLAND
In the meantime, Otto Frisch had not sat still; he had immigrated to England and was now working with Rudolf Peierls at the University of Birmingham. Peierls had previously worked for Fermi at the University of Rome. Frisch and Peierls collaborated on the problem of how much U-235 would be needed for a bomb. This was later referred to as the critical size. To their surprise, their calculations indicated that a very large amount was needed. Despite the setback, their work showed that a bomb was indeed possible. In fact, they concluded in their report that what was needed was two pieces of U-235 that were smaller than the critical size. When the two pieces were brought together, they would immediately explode, but they could be handled safely as long as they were below the critical size. Because of their work, British officials decided to set up an organization called the MAUD Committee for atomic research in early 1941. No one was quite sure where the name came from. It is not an acronym, and it appeared to have come from a letter sent by Meitner to an English friend. She ended the letter with the name Maud, and for a while people thought it was some sort of code. It turned out that it wasn't.
In July 1941 the MAUD Committee issued two reports.13 The first stated that it had been determined that a bomb could now be built using approximately twenty-five pounds of enriched uranium, and it would have the destructive effect of eighteen hundred tons of TNT. It recommended that work begin immediately and that it should be conducted in collaboration with the United States. At the time the United States had many more resources than England for building such a bomb. In addition, a new committee codenamed Tube Alloys was set up in conjunction with Canada for further developments of nuclear weapons.
In June 1940 the MAUD report was sent to Vannevar Bush, the head of the National Defense Research Committee in the United States. The results were reported to Roosevelt, and there was a considerable amount of discussion about the possibility of a bomb, but little action.
Finally, in August 1941, Mark Oliphant, one of the leaders of the MAUD Committee, decided to fly to United States to see what the problem was. To his dismay, he found that Bush had done very little with the report; it was sitting in a safe. He immediately met with several members of the American Uranium Committee and stressed the importance of action. He met with Ernest Lawrence on September 21, and they were soon joined by Robert Oppenheimer, who was surprised by what the British had achieved in relation to the bomb.
Both Oppenheimer and Lawrence were now committed. They contacted Arthur Compton of the University of Chicago, and a review committee was immediately set up. There was still some skepticism about U-235, but in the meantime Glenn Seaborg of the University of California had managed to create element 94 (now called plutonium). And on May 18 they showed that it had a rate of fission nearly twice that of U-235. It was therefore also a suitable material for an atomic bomb. This was good news. If a reactor could be built, this new element could be produced relatively easily.
In the meantime the MAUD Committee had sent further reports to United States. The second and third reports came in late October 1941, and they were much more urgent. They reported that a mass of about twelve kilograms was all that was needed for criticality.
Then, on December 7, 1941, planes from several Japanese aircraft carriers bombed the American fleet at Pearl Harbor. The next day Roosevelt declared war on Japan, and soon the United States was at war with Germany and Italy as well.
HEISENBERG AND BOHR
By December 1940, Heisenberg and his team had built their first reactor. It was a relatively simple device that failed to initiate a chain reaction. But other similar experiments were going on in Heidelberg and Leipzig. The one in Leipzig used paraffin wax as a moderator, and the one in Heidelberg used heavy water. Both experiments again failed. The problem appeared to be due to the uranium; it was not enriched enough with U-235. By now the Germans knew that if element 94 could be produced in a reactor, it would also make an excellent material for a bomb. So there was even more incentive to accelerate the project, and for this they needed more heavy water. Furthermore, they were now beginning to worry about the progress being made in England and the United States. Heisenberg was certain that his group was far ahead of them, but he had to know for sure.14
Denmark had been occupied by Germany in April 1940, but Bohr had decided to remain at his Institute for Theoretical Physics in Copenhagen. He was of Jewish descent, but the Danish government had, as part of its surrender, demanded that the Jews of Denmark be unharmed. Bohr would know what was going on in relation to the English and American projects, but how could Heisenberg talk to him? Both men were under close scrutiny.
When the Germans took over Denmark they set up a German cultural institute in Copenhagen. A proposal was given to the German foreign office to set up a symposium on theoretical physics and invite both Heisenberg and Bohr. It was set up for mid-September 1941. Heisenberg was anxious to talk to Bohr, but he worried about what his reaction might be. He had worked with Bohr and been on friendly terms with him for years, but now things were quite different. For his part, Bohr was not anxious to attend the conference, and he boycotted most of it. He was curious, however, about how much progress the Germans had made on atomic-bomb development, and he knew Heisenberg was part of the program.
Heisenberg visited Bohr's institute and had lunch with him twice. Bohr was quickly turned off, however, by Heisenberg. He told Bohr that it was critical that Germany win the war, as it would be helpful for the development of Eastern Europe. Bohr could not agree less. In their second meeting Bohr asked about the German atomic bomb and what progress had been made. Heisenberg knew the Gestapo, the secret police of Nazi Germany, was watching his every move, so he suggested they move to Bohr's study.
It soon became clear to Bohr that Heisenberg was doing everything possible to help Germany develop a bomb. This was a shock to Bohr. Heisenberg proceeded to make a sketch for Bohr. Bohr thought it was a drawing of an atomic bomb, and he was repulsed. As it turned out, it was actually a sketch of a reactor. Then Heisenberg started to probe him about the progress the English and Americans had made. Bohr was immediately suspicious that Heisenberg was trying to probe for secrets. Bohr said little, and the meeting, for the most part, was a disaster for both men.
THE MANHATTAN PROJECT
On October 9, 1941, Roosevelt gave the go-ahead for the development of the atomic bomb, and on December 6, the day before the bombing of Pearl Harbor, he authorized what would eventually become known as the Manhattan Project. Various projects were set up in laboratories across the United States, but there was little coordination, and many of the people involved began to get frustrated with the progress. Something had to be done.
Vannevar Bush, the director of the Office of Scientific Research and Development, suggested that a single person should be in charge and that the entire project should be directed by the Army Corps of Engineers. For many of the scientists this was not good news. They didn't like the idea of being bossed around by army officials. Bush selected Leslie Groves, a no-nonsense colonel with considerable experience in directing military construction projects, as military director of the Manhattan Project. Groves was not happy to take on the project at first.15 He knew little about physics and didn't have much faith that such a bomb would work. Furthermore, he wasn't popular with the men who had worked under him because of his gruff approach, but everyone admitted that he got things done. And, as it turned out, he was the ideal man for the job.
Within a few weeks of taking his new assignment, at which point he'd been promoted to the rank of brigadier general, Groves went on an inspection tour of the various facilities throughout the country that were involved in the project. He traveled to Columbia University and talked to Harold Urey, who was involved with in the effort to separate U-235 from natural uranium, then he went to the University of Chicago to meet with Fermi. Fermi was now involved in building the first reactor. Then he went to the University of California at Berkeley, where he met Lawrence, who was then building a large particle accelerator called a cyclotron. He was impressed with some of what he saw but disheartened by other parts of the project. The basic problem seemed to be that there was no real organization and cooperation, and no sense of urgency.
At Berkeley he talked to Robert Oppenheimer, and almost from the moment he met him, he was impressed. Oppenheimer had a good overall grasp of what was needed to achieve the goal of producing a bomb, and he had considerable confidence that it could be done. His enthusiasm and confidence appealed to Groves. Groves had originally thought that he would select Lawrence as the scientific director of the project, but after meeting Oppenheimer he changed his mind. He was now sure that Oppenheimer was his man, and he suggested him at the next meeting of the Military Policy Committee.16 After a few checks on him, however, it became obvious that there were problems. Everyone agreed that he was a first-rate scientist, but he had no experience in directing people. In addition, a check by the Federal Bureau of Investigation indicated that he might be a security risk, as some of his closest friends, including his brother, had been associated with the Communist Party. The FBI told Groves to find someone else. Groves went back and checked on other possibilities, but he came back even more convinced that Oppenheimer was the best man for the job. Stubbornly, he resubmitted Oppenheimer's name, and after some arguments, Oppenheimer was finally accepted.
Groves began conferring with Oppenheimer on how to approach the problem. Oppenheimer suggested that all the scientists should be brought together in a single lab or complex, and, as it turned out, this was what Groves had also been thinking. They would need a place that was relatively isolated so that the facility would not draw a lot of attention. Oppenheimer had spent much of his earlier life in northern New Mexico, and he thought it had all the qualifications needed. He remembered a place near Jemez Springs, about thirty miles north of Santa Fe. He had recovered from tuberculosis there in the summer of 1928. It seemed like an ideal place. A school called the Los Alamos Ranch School had been established there but was now on the verge of bankruptcy. Groves visited the area and agreed with Oppenheimer. He purchased the school and surrounding area immediately.
The project did not get off on a good track at first, however. Oppenheimer had thought that a group of about thirty scientists would be enough, and he was sure that there would be no problem directing them. Almost immediately Oppenheimer and Lawrence began scouring the country for the best scientists to bring to the new site. Some were reluctant to go, not sure if they would like the remoteness, isolation, and secrecy that would be required. Furthermore, the army was running things, in particular, the construction of the town at the site and the laboratories that would be needed. Something else that was bothersome to them was that Groves wanted compartmentalization. In short, he wanted each group to know everything about the particular aspect of the bomb they were working on but little or nothing about what other groups were doing. The fewer the people that knew everything about the project, the better. And secrecy had to be of the highest order; there would be no publication of any discoveries. Scientists did not normally work this way.
All of this became a problem for Oppenheimer, and his original group of thirty scientists grew to one hundred and then to fifteen hundred. For the first few months the place was a mess. Buildings, laboratories, roads, and many other facilities were being built, and with the spring thaw there was mud everywhere. It was his job to keep everybody happy. He had recruited some of the best physicist in the world, including Edward Teller, Hans Bethe, Felix Bloch, Richard Feynman, and Robert Serber, and he had lined up Enrico Fermi and Isidor Isaac Rabi as consultants (they were already involved in important war projects).
The problem before them seemed straightforward enough: get enough enriched uranium (high in U-235) to create a critical mass, and at the proper time bring two sub-critical masses together to create a chain reaction. The first calculations of the critical mass needed were not encouraging; it appeared to be very large, perhaps too large to be carried in an airplane as a bomb. But innovations were made so that the neutrons created in the blast were reflected back into the blast by a shield. This reduced the critical mass to about thirty-three pounds of U-235. At the same time it had now been shown that a bomb could also be made from plutonium, and only eleven pounds of plutonium would be needed. Of course, a reactor would have to be built to get the plutonium, so most of the interest was still in U-235.
In reality a slightly greater amount than the critical mass would be needed because of various problems; it was usually referred to as the super-critical mass. When two sub-super-critical masses were brought together they would create an explosive force equivalent to twenty thousand tons of TNT. But there was a serious problem: the masses had to be brought together very rapidly. If they came together too slowly, some of the mass would fission and detonate, which would blow other parts of the mass apart before they could fission. Calculations showed that they had to be brought together at a speed of 3,300 feet per second. However, this was beyond the highest speed produced by any explosive technique; the highest artillery speed known at the time was about 3,100 feet per second.
Furthermore, there was another problem, and it was associated with the neutrons that would be triggering the fission. All that was needed was one neutron to start the chain reaction, but it had to be delivered exactly when the two halves came together. The problem was that there were neutrons all around; in particular, they were generated by cosmic rays that came from space and continually struck the earth. They were not actually rays (or radiation); they were mostly particles of various types, including nuclei of various elements and protons and electrons. But when they struck our atmosphere they produced neutrons, and these neutrons could trigger the uranium (or plutonium) prematurely. So the bomb had to be shielded from them.
Nevertheless, the bomb needed a proper and reliable source of neutrons at exactly the right time. A “gun” design was devised to bring together the sub-critical pieces and the neutrons all at the same time. But this design was plagued by problems, so another solution was put forward that made use of an implosion. The idea in this case was to construct a sphere of separated plugs of uranium that could be forced together using conventional explosives that would be placed behind them. When all the pieces came together it would explode. Again, there were problems, and just as troubling was the fact that so far a simple reactor had not even been built.
THE FIRST REACTOR
Construction of the first nuclear reactor began in October 1942. A nuclear reactor is a slowed-down version of an atomic bomb; it was needed to verify that a nuclear chain reaction would, indeed, occur, and that a bomb was possible. Enrico Fermi, as the foremost expert in the world on neutrons and neutron bombardment, was put in charge of the project. The reactor was built in the racquet courts beneath the bleachers of the football field at the University of Chicago. It consisted of seventy-six layers of graphite bricks, each of which measured four inches by four inches by twelve inches. Because of this layering, it was referred to as a pile. Scaffolding was eventually constructed around it as it began to grow, so that the upper layers could easily be reached. It was made up by piling two layers of pure graphite bricks, then two layers of bricks loaded with uranium. Cadmium rods were also inserted in the pile. Cadmium is a strong absorber of neutrons, and the cadmium rods would help keep any reaction that occurred under control. They could easily be raised and lowered.17
Fermi had two main assistant scientists: Herbert Anderson and Walter Zinn. Each man led a group that worked twelve-hour hour shifts. So work went on around the clock. As the pile was built up, the number of neutrons emitted was carefully monitored. Neutron counters were placed within the pile to do this. A factor called k gave a measure of the number of neutrons that were being generated within the reactor. When k was 1.0, the pile became critical so that the fission reaction was self-sustaining. Fermi wanted to increase it just above 1.0, but he didn't want it to go any larger. If it did, everything could get out of control and an explosion could occur.
Late in the day on December 1, 1942, k was very close to 1.0, and it appeared that criticality would be reached the next day. The next morning a large crowd formed on the balcony that overlooked the reactor. Fermi told his assistant to pull one of the cadmium rods out from the pile slowly. As he pulled it, the clicks in the neutron counters increased rapidly. As he had done throughout its construction, Fermi made some quick calculations using a small slide rule. He then ordered his assistant to pull the rod out a little farther, and again the clicking rate increased.

Details of a simple reactor.
Everyone was waiting in anticipation, and to their surprise Fermi decided to break for lunch. After lunch they reassembled and Fermi again told his assistant to pull the cadmium rod out of farther. Suddenly the counters went wild. The pile had gone critical. Fermi allowed it to continue clicking wildly for several minutes, then he ordered his assistant to push the rod in to shut it down.
Most scientists now regard this as the beginning of the atomic age. It was the first working nuclear reactor; nevertheless, there was still a long ways to go to get an atomic bomb. But now there was no doubt that it could be built.
THE CONTINUING MANHATTAN PROJECT
Work on the Manhattan Project had started. The major problem was separating U-235 from natural uranium. The uranium nucleus would fission because it was so large and unstable, and it tended to break in half easily. The two isotopes of uranium each had 92 protons, but U-238 had 146 neutrons and U-235, which was the type that fissioned easily, had 143 neutrons. When a neutron was projected at U-235, its nuclei would break down into barium and krypton, and most importantly, when it split, it would release other neutrons that would go on to split other nuclei. The problem was that less than 1 percent of natural uranium was U-235. For the bomb, U-235 was needed, or at least very enriched uranium (uranium that consisted mostly of U-235).18
Three methods were known for separating, or enriching, uranium: gaseous diffusion, thermal diffusion, and what was called the electromagnetic method. In the case of gaseous diffusion, natural uranium is passed through some type of porous medium. The heavier nuclei of U-238 will gradually be left behind, and the resulting material gradually increases its percentage of U-235. In this method, uranium is combined with fluorine to form a fluoride gas. Diffusion technology at the time allowed the separation of only micrograms of enriched uranium. So it was obvious that it would have to be done on a very large scale to get enough enriched uranium for a bomb in a reasonable amount of time. The plant was set up at Oak Ridge, Tennessee, in 1943; it was called K-25, and no one working there knew what it was for. Everything was kept secret. Chrysler built the huge diffusers needed, and a problem soon developed. The diffusers had to be built of nickel, and nickel was in short supply, but Chrysler soon devised a way around the problem.
The overall plant was huge, covering an area of two million square feet (half a mile long by four hundred feet wide). The gas passed through ten thousand miles of tubing before it was enriched enough to use in the bomb. About fourteen pounds of enriched uranium were produced from each ton of uranium ore.
The second method of enrichment was called the electromagnetic method. It was discovered at the University of California at Berkeley by Lawrence and his team, and it required the new cyclotron, or atom smasher, that Lawrence had just built. Groves had little confidence in the method because it produced only micrograms of enriched material. Nevertheless, he gave the go-ahead for the work as a backup to the gaseous-diffusion plant, just in case gaseous diffusion didn't work. The electromagnetic plant was also set up at Oak Ridge, and it was called Y-12. Again, it was a huge plant, almost as large as the gaseous-diffusion plant, and, again, none of the workers knew what it was for.
But even with these two programs, things were still going too slow for Groves. He decided to set up a thermal-diffusion plant at Oak Ridge also. Amazingly, it was built in only sixty-nine days. Again, it did not produce very much enriched uranium, but it was soon discovered that if the material from the electromagnetic plant was fed into the thermal plant, the process was much more efficient.
While all this was going on, Groves had another backup. In late 1942 Fermi had shown that a nuclear reactor could be built. And it was soon well known that plutonium could be produced in a reactor from U-238, and that plutonium was also a fissionable nucleus. Furthermore, relatively pure plutonium could be produced at a greater rate than U-235 production. So Groves ordered the construction of three nuclear reactors in Hanford, Washington. They had the code name X-10. The problem at this stage was that only one relatively small reactor had been built, and the ones in Hanford would have to be huge in comparison, so the technology had to be developed fast. The reactors were built under the direction of Gilbert Church, and strangely, he had no idea what they were going to be used for. He brought in forty-five thousand workers from across the country, and none of them were told what the devices were, or what they were for.
Finally, in early 1945 things began to look up. Considerable amounts of enriched uranium were being produced as well as significant amounts of plutonium. Within months there was enough uranium for a bomb and enough plutonium for several bombs.
While all this was going on, work at Los Alamos was continuing. One by one the problems were being overcome. It was now known how much uranium or plutonium would be needed for a critical mass. Considerable work had been done on both designs for bringing the sub-critical masses together: the gun design and the implosion method. It was, in fact, shown that the gun design would not work with plutonium. Even when U-235 was used for the gun design, it did not appear to work as well as the implosion method. Calculations showed that the implosion would squeeze the masses to super-critical density without the need for a super-critical mass. Furthermore, conventional explosions could be used for bringing the sub-critical plugs together.
Two bombs were developed, referred to as Fat Man (FM) and Little Boy (LB). Little Boy was made with enriched uranium, and Fat Man was made using plutonium. More plutonium than uranium was available at this time, so the initial tests were done using a plutonium bomb.
TRINITY
Things took a strange turn in April 1945. On April 12, Franklin Roosevelt, who had been a strong supporter of the bomb, died, and Harry Truman took over as the thirty-third president of the United States. Strangely, Roosevelt had told him very little about the construction of the atomic bomb, but he did know about the existence of the Manhattan Project. No one knew what to expect of Truman. But, as it turned out, he was up to the job. The war in Germany was over within a few weeks, so the bomb would obviously not be needed there. Japan, however, was a holdout, and it appeared as if it might hold out for a long time.
Before a decision could be made about whether to use the bomb, however, it had to be tested to make sure it actually worked. The test site was called Trinity; it was about sixty miles northwest of Alamogordo, New Mexico, on a desolate stretch of desert. At point zero (the actual bomb site) a 110-foot tower was constructed; the bomb would be placed at the top of the tower. A concrete command center was built approximately ten thousand yards away; several other bunkers were also constructed in the area. A large number of instruments were also scattered around the area to measure the impact of the blast.19
The bomb itself was to be a plutonium bomb in which about eleven pounds of plutonium were used. The “ball” of plutonium would be about the size of a small orange. The test was originally scheduled for July 4, but problems developed and it was rescheduled for July 16. Oppenheimer insisted that a dry run (without an explosion) take place before the actual test. It was scheduled for July 14, and to Oppenheimer's dismay a problem was detected. The case for the explosive device was slightly cracked and pitted. Oppenheimer was worried that it would cause a problem, but it seemed too late to call off the test scheduled for July 16. Everything was rechecked; Hans Bethe went through every aspect of the device carefully to make sure that there were no problems. There were, however, a number of uncertainties; the major one was the energy that would be produced by the blast. No one was certain what it would be; estimates ranged from a blast equivalent to forty-five thousand tons of TNT down to one equivalent to only a thousand tons.
The blast was to take place at 5:30 a.m. on the morning of July 16. Within a few hours of time zero a thunderstorm struck and it began to rain. Finally, however, the rain stopped and the sky cleared, so it appeared as if it would go off as scheduled. All observers were equipped with welder's glasses to protect their eyes. The countdown began just before 5:30 a.m. As the countdown reached zero everyone held their breath in anticipation. Suddenly a small bright region erupted close to the horizon. Within a few seconds it had grown into an awesome spectacle: a huge red sphere that was too bright to look at directly. Everyone was silent; then came the blast, followed by a long rumble. At first there was complete silence among the spectators, then several sighs of relief. It had worked. Fermi was quietly busy performing a simple experiment: he dropped several small pieces of paper to see how far they were carried by the shockwave. This would give an estimate of the energy produced. He soon showed that it was equivalent to about ten thousand tons of TNT. News of the success was sent immediately to President Truman.
THE GERMAN BOMB
There was now no doubt: the Americans, with the help of the British, had beaten the Germans to the atomic bomb. But what had happened to the German project? There's no doubt that Hitler wanted super weapons, including the atomic bomb. He boasted about them frequently, but as Germany began to lose the war, he wanted everything as fast as possible, and the V-2 rockets looked like they could be produced much faster than the atomic bomb, so most of his attention was directed toward rocket development. He eventually began to lose interest in funding the atomic-bomb project, so little money was made available. Nevertheless, an active program continued until near the end of the war. By 1943, however, Allied raids on Berlin were increasing rapidly, forcing relocation of the project's major parts to southwestern Germany.
The Americans and British were still worried, however, about how far along the German program was. After all, the Germans had had a better start, with the discovery of fission having taken place in Berlin. Because of this, Grove set up a group of scientists and military officers in September 1943 called the Alsos Mission. Its purpose was to follow the Allies, as they moved through Italy, France, and Germany, to find out as much as possible about the German bomb project and any other similar projects. The group consisted of thirty-three scientists and seven military officers. It was commanded by Colonel Boris Pash with Dr. Samuel Goudsmit as head of the scientific group. They were to capture critical Germans physicists and find any uranium that the Germans might have stockpiled.
For the most part, they followed as closely as possible behind the front lines, but in a number of cases they actually crossed it and came under fire. They soon discovered that about one thousand tons of uranium ore had been shipped to Germany and distributed to several labs in Germany and occupied France. They also found documents and other information at Strasbourg University indicating that there were laboratories related to nuclear research at Haigerloch, Hechingen, and Tailfingen in southwest Germany. There was a problem, however; the Russians were now pushing into Germany from the east, and the French now also had an army that was pushing in the direction of southwest Germany. Groves and other top military brass didn't want the nuclear research sites falling into Soviet hands, or even French hands, before they got to it.
Pash appealed to the top American general to push toward the southwest, but he was told that a deal had already been made with the French. The French would occupy that region, and he would have to get permission from the French to enter it. Pash was annoyed; nevertheless, he set off toward Hechingen and managed to bluff his way past some of the French guards, but his group was stopped before they got there. Again, he had to argue with another French officer, but he was finally allowed to pass.
On the morning of April 24, Colonel Pash and his group finally reached Hechingen. He was surprised to discover that there was still a group of German soldiers in the area, and an hour-long firefight ensued. Finally, his group entered the small town and began looking for the nuclear lab. They soon found Heisenberg's office and lab, and they captured several important scientists, but Heisenberg had already left. His reactor, however, was discovered a few miles away in a cave in the nearby town of Haigerloch. It was beneath a church. The reactor was cylindrical and made up of graphite blocks; the uranium, however, was missing, along with the heavy water that had been used. Nearby, however, the group discovered three drums of heavy water and one and a half tons of uranium ingots buried in a nearby field.
But Heisenberg was still missing. Pushing on, Pash and his men found Heisenberg at his home, waiting for them. The team back at the cave took everything they wanted out of it and set charges to blow it up. Church officials, however, pleaded with them not to detonate the explosives, explaining that the explosion would destroy the church and castle above the cave. So they left it intact.
It was soon obvious that the Germans had made little progress toward the bomb. Heisenberg was still trying to get a nuclear reactor to work, and without it there could be no bomb.
DECISION TO USE THE BOMB ON JAPAN
The Trinity test had shown that the bomb worked. But the war with Germany was over, so it could not be used there. The war with Japan, however, was far from over, although there was no doubt that US forces were winning and that Japan would eventually be occupied. So the question was, should the United States use it, and if so, what cities should be targeted? As expected, there were arguments from both sides. The Japanese bombing of Pearl Harbor, and the stubbornness of the Japanese at Okinawa and Iwo Jima and other places in the Pacific, showed that surrender was a foreign word to them; they would fight to the last man. Furthermore, Tokyo had been firebombed almost to oblivion, yet the Japanese continued to fight. The only alternative, it seemed, was an invasion of the homeland, and few wanted that because it was obvious that a lot of American lives would be lost in the effort.20
Many people, however, worried about the ramifications of dropping an atomic bomb. Szilard was one of the most vocal. He tried desperately to meet with President Truman; he even sent a petition to him that was signed by fifty-three scientists. He urged the president to demonstrate the bomb to the Japanese first. Truman apparently looked closely at both sides of the argument and decided to go ahead with the bombing. After all, air raids on Japan using conventional bombs had already produced devastating effects equivalent to twenty thousand tons of TNT. This was about equivalent to the force of a single atomic bomb. And the Japanese still had not surrendered.
Two atomic bombs were therefore dropped, the first on Hiroshima on August 6, 1945, and the second on Nagasaki on August 9. A few days later Japan finally surrendered.