Geologic Storage
The Permian Basin in West Texas is the major oil-producing region in the United States. Oil fields that have been producing crude oil for decades slow down and produce less oil each year, but a large fraction of the original oil remains trapped in the pores of the rock. To continue extracting more oil from these existing reservoirs, the oil industry is always trying to develop Enhanced Oil Recovery (EOR) technologies. One such EOR technology, CO2 flooding, was first used in 1972 in Scurry County, Texas. CO2 injected into the oil reservoir helps mobilize the trapped oil so it can flow to a production well. Eventually the CO2 will break through and flow up a production well, where it will be separated from the oil for reinjection into the reservoir. Today, the Permian Basin has over 70 CO2 EOR projects and is crisscrossed by over 4000 km of CO2 pipelines. Oil field operators purchase and inject tens of millions of tons of CO2 a year.5
The Permian Basin is by far the world’s largest collection of CO2 EOR operations because they have access to cheap CO2 from natural reservoirs. Most gas reservoirs are primarily methane, with varying amounts of other gases, including CO2. In the western United States, there are a number of large gas reservoirs that contain nearly pure CO2, including Bravo Dome in New Mexico, and McElmo Dome and Sheep Mountain in Colorado. Pipelines bring the CO2 to the oil producing fields of the Permian Basin. It is this cheap CO2, generally delivered for under $20/tCO2, which makes these EOR operations successful.6 Additionally, as will be discussed in the next chapter, some anthropogenic CO2 sources using carbon capture feed their CO2 into this pipeline network to help meet the growing demand for CO2 EOR.
Using CO2 to help produce CO2-emitting oil will not solve the climate change problem by itself, but it has made significant contributions to CCS. Most of the injected CO2 will remain in the reservoir after oil production ceases, significantly lowering the net carbon intensity of the oil produced. More important though, EOR has developed the operational methods and history to show that large amounts of CO2 can be injected safely into geologic formations. These EOR projects have financed a CO2 pipeline network and helped develop CO2 handling technology. This knowledge and infrastructure were instrumental to many CCS projects that followed. As such, CO2 EOR has become an important stepping-stone for the development and deployment of CCS.
Target Formations
A good geologic storage formation for CO2 must meet four main criteria. First, the target formation must be porous with good permeability. A bucket of sand meets this criterion. If you add water to the bucket, the water easily flows through the sand because of its high permeability, its pores the spaces between the grains that the water fills. Deep in the Earth, sandstone formations, found around the world in sedimentary basins, exhibit similar characteristics to our bucket of sand. Second, the target formation must be below 800 m depth. To ensure that the CO2 remains in a dense liquid-like phase, it needs to be stored at pressures greater than its critical pressure of 73.9 bar. The average hydrostatic pressure at 800 m depth is 80 bar, so anything deeper will safely satisfy this criterion. For CO2 storage reservoirs, typical depths are 2–3 km, with pressures of 200–300 bar and temperatures of 60–100°C. This results in a range of specific gravities for the CO2, from 0.5 to 0.8. Compare this to a CO2 gas with a specific gravity of about 0.001 or to liquid water with a specific gravity of one: being much denser than a gas, much more CO2 can be stored in the pore space of the formation. Being less dense than water means that the CO2 is buoyant and will want to rise up in the formation. This leads to the next criteria: the target formation must have an impermeable caprock. Since the CO2 is buoyant, an impermeable caprock will keep it trapped in the target formation. Thick shale layers, consisting primarily of clay, make an excellent caprock. Finally, it is desirable for a good storage formation to be thick and continuous over large areas in order to be able to store large volumes of CO2.
The geological formations that meet the above criteria fall into two categories: oil and gas reservoirs, or deep saline formations. Oil and gas reservoirs have proved that they can hold hydrocarbons for millions of years. This bolsters confidence that they can store CO2 for a long time. Because they have been producing hydrocarbons, their flow characteristics are well known. Questions do arise about whether the wells drilled into the reservoirs and the removal of the hydrocarbons have compromised their integrity. Most capacity lies with depleted oil and gas reservoirs, but active oil reservoirs have become a high-priority target for CCS because of the income generated by EOR. Deep saline formations are filled with salty water (i.e., saline) and are much deeper in the Earth than drinking water aquifers. Because they have little or no economic value, very few wells have been drilled into them, making the data regarding their physical characteristics sparse. Ultimately, these deep saline formations will store the most CO2 because they are widespread and have much larger volumes than oil and gas reservoirs.
Trapping Mechanisms
Geologic storage of CO2 is the mirror image of oil and gas production. Instead of drilling wells into the Earth to extract oil and gas, wells are drilled to inject CO2. Injection requires the CO2 to be pressurized, typically in the range of 100–150 bar. As it moves down the well, the pressure will rise due to weight of the CO2 in the pipe. For injection, the pressure in the pipe must be higher than the formation pressure, so that at the perforated interval at the bottom of the well, the pressure pushes the CO2 into the formation, where it displaces water and moves into the pores of the rock, forming a plume.
Once the CO2 enters into the formation, the laws of nature take over and determine its fate. The better we can characterize a formation in terms of its structure, dimensions, and physical properties, the better we can model how the CO2 will move through the formation and what will happen to the plume over time. The formation will experience a pressure rise associated with the injected CO2. The magnitude of the pressure rise will depend on the formation properties, both in the area of the plume and far away, where the formation is compressed to accommodate water displaced by the CO2. The section below, on induced seismicity, will explore the implications of this pressure rise.
Four main mechanisms, working together, trap the CO2 in the formation: structural trapping, capillary trapping, solubility trapping, and mineral trapping.7 Structural trapping refers to the CO2 being beneath the formation’s impermeable caprock. As the CO2 plume rises, it will eventually run into the caprock, which will stop its rise and keep the plume in the formation. The geometry of the caprock can form a trap, like an inverted bowl or hat, which then fills with CO2 and limits the lateral extent of the plume.
Capillary trapping, sometimes referred to as residual trapping, refers to the CO2 being immobilized in the pore space as the plume moves through the formation. It is a function of water and CO2 competing to move though the small pores between sand grains. A capillary trapping situation that most of us can relate to is dripping oil on our shirt while eating. You can try to rub out the stain using water, but that will not work. The oil is trapped in the holes between cloth fibers. In this sense, capillary trapping is a very secure storage mechanism.
Solubility trapping refers to the dissolution of CO2 into the formation water. Some CO2 dissolves rapidly at first contact with water at the plume edge, saturating the water. Behind the plume front, no more CO2 can dissolve unless new unsaturated water encounters the CO2. The CO2-saturated water is denser than unsaturated water, meaning it will sink, which is one way of bringing more unsaturated water in contact with the CO2. Increasing the amount of dissolved CO2 increases storage security.
Mineral trapping refers to the reaction of formation minerals with dissolved CO2 to incorporate the CO2 into new minerals, most commonly calcite. The rate and ultimate amount of mineral trapping depends on the rate of dissolution of CO2, and the availability of reactive minerals in the formation. Most sandstones meet both needs only to a limited extent. Mineral trapping removes CO2 from the plume very slowly. However, as discussed in following sections, some rock types such as basalt contain reactive minerals, and dissolving CO2 prior to injection can accelerate reactions.
The trapping mechanisms discussed above create storage security. The multiple barriers relying on different physics are additive, so that even if one system is flawed (e.g., the caprock), most of the CO2 will be retained in the formation.
Storage Security and Monitoring
The number one question people ask about geologic CO2 storage is: “Will it leak?” The IPCC, in typical bureaucratic language, answers as follows: “Observations from engineered and natural analogues as well as models suggest that the fraction retained in appropriately selected and managed geological reservoirs is very likely to exceed 99% over 100 years and is likely to exceed 99% over 1000 years.”8 The IPCC defines very likely as 90–99 percent and likely as 66–90 percent. Allow me to translate: if done properly, there will be no significant leakage. So why the longwinded statement? First, as seen for all types of projects, not all of them are “appropriately managed.” Second, the selection of an appropriate reservoir for geologic storage is crucial to success. Finally, since geologists think on geological timescales, they will not guarantee that a fluid injected in the ground will never come out; there are simply too many changes in Earth systems on geologic timescales for that. However, if an appropriate reservoir is selected, and if the project is appropriately managed, we should expect little or no leakage over human timescales of interest.
It is important to understand the nature of any potential leakage. The CO2 is being injected into a porous medium, as opposed to a big cavity in the Earth. Therefore, if leakage occurs, it is more like squeezing a wet sponge rather than popping a water balloon. This means the chances of a large and sustained rapid release of CO2 are small. Leakage would only happen if the CO2 found a pathway from the formation to the surface. Based on decades of injection and EOR experience, wells drilled into or through the storage formation are the most likely failure points. While wells are designed to isolate fluids (such as oil, gas and brine) in one zone from fluids (such as freshwater) in other zones, engineering failures have occurred. That is why the US EPA requires a survey and, if needed, remediation of all wells before it will issue a permit for any injection, including geologic storage of CO2. A second potential pathway is a geologic flaw in the caprock. Certain types of faults can provide these, and a good characterization of a reservoir should be able to detect the existence of faults. Fracturing the caprock could also open up pathways for CO2 to escape, but this will only happen if the pressure rise from the injection gets too large. Properly managed projects will monitor formation pressure and adjust operations to keep the formation pressure within safe limits.
Since maintaining proper formation pressure is critical and CO2 injection can increase formation pressure, the greatest risk for problems to occur is near the injection wells while active injection is taking place. Once the injection stops, the pressure will start to drop back down as the pressure equalizes throughout the formation. Models can help design systems to stay within safe pressure limits. However, there will still be uncertainty in understanding the formation properties, so monitoring the pressure is not only crucial, but also required by EPA, to adjust operations if necessary.
Both during and after injection, numerous monitoring techniques are available to ensure sites are both appropriately selected and managed. Making direct measurements of pressure and temperature involves putting instruments at or down the injection well. Projects may also drill observation wells to take measurements at other locations in the formation. A number of seismic techniques allows one to image the CO2 in the formation. Seismic techniques involve sending sound waves into the Earth; the waves will travel at different speeds, depending of the density of the medium through which they are traveling. Since the density of CO2-filled rock is different from brine-filled rock, it allows one to locate the CO2 plume. Numerous other monitoring techniques, including surface monitoring to detect any CO2 that migrates to the surface, are also available. In putting together a monitoring program for a project, there are trade-offs between the level of monitoring versus its cost. Initial demonstration projects usually have extensive monitoring programs in order to gather as much information as possible to improve the knowledge on how CO2 behaves in the subsurface. Commercial projects are developing more targeted monitoring packages to comply with regulatory requirements for safely injecting the CO2.
There is a debate about how long to monitor a formation after injection stops. The US EPA regulations for CO2 storage wells have set a default time of fifty years post-injection monitoring, with possibilities to reduce the time based on site-specific data and modeling. Many in industry and elsewhere feel that this requirement is excessive and shorter post-injection monitoring periods are adequate. Impacts of elevated CO2 levels are described below:
At low concentrations (less than 1% by volume), CO2 causes no ill effects on humans, fauna or flora. In fact, CO2 is essential for life, being a critical component in photosynthesis. Some greenhouses purposely elevate CO2 levels in order to “fertilize” the plants. At concentrations of about 6% by volume, CO2 can cause nausea, vomiting, diarrhea, and irritation to mucous membranes, skin lesions and sweating. At about 10% by volume, it will cause asphyxiation.9
Can CO2 escaping from geologic storage reservoirs reach the elevated concentrations that cause harmful impacts? Under most cases, the answer is no. Even at high leakage rates, CO2 leaking from the reservoir will disperse into the atmosphere and not reach harmful concentrations. However, there are two major exceptions. CO2 is heavier than air, so it can gather in low-lying areas on still days. If a big enough leak occurs in a topography that will gather the CO2, it does present a risk. The second exception is enclosed structures, such as the basement of a house. This scenario is analogous to the way radon can build up in basements that are not adequately ventilated. In both of these cases, monitoring can help detect the presence of CO2. In general, the biggest impact of CO2 leaking from geologic storage will be the ineffectiveness of the money spent to keep that CO2 out of the atmosphere. To date, there has been no significant leakage reported from any operating CCS projects.
Induced Seismicity
Induced seismicity refers to human-made triggering of seismic energy. Injection or withdrawals of any fluid that changes pressure within the Earth can trigger audible adjustment of rock grains and layers. Instruments can locate and measure these noises, known as microseismic events. These are small and of no concern. In fact, they are helpful in providing data about the subsurface response to injection. What is concerning is when pressure changes work to augment the stresses already in the Earth, triggering larger adjustments of rocks and large movements that are felt by people as earthquakes and that can cause damage. Large events are associated with only a very small percentage of injections, but they are unacceptable.
In 1966 at the Rocky Mountain Arsenal in Denver, Colorado, one of the first recorded instances of induced seismicity occurred as a result of disposal of contaminated fluids.10 Around this time there was a whole series of earthquakes in the Denver area, with the largest being a magnitude of 5.3. While not all the seismic activity could be connected to the fluid injections, this incident was the first to raise the issue of induced seismicity.
More recently, the injection of wastewater from oil and gas production operations has raised the induced seismicity concern:
Between the years 1973–2008, there was an average of 21 earthquakes of magnitude three and larger in the central and eastern United States. This rate jumped to an average of 99 [magnitude] 3+ earthquakes per year in 2009–2013, and the rate continues to rise. In 2014, alone, there were 659 [magnitude] 3 and larger earthquakes. Most of these earthquakes are in the magnitude 3–4 range, large enough to have been felt by many people, yet small enough to rarely cause damage. There were reports of damage from some of the larger events, including the [magnitude] 5.6 Prague, Oklahoma earthquake and the [magnitude] 5.3 Trinidad, Colorado earthquake.11
Risks of triggering large seismic events can be managed by selecting and properly characterizing appropriate formations and controlling pressure changes. Techniques for controlling pressure are primarily selecting formations that can accept large injection rates and limiting the injection rate in each well. Removing saline water from the formation can limit pressure rise. The biggest problem with this technique is figuring out what to do with the saline water, which can be a very nasty fluid. However, it is quite likely that new technologies will make some form of active reservoir management practical in the future.
Storage Capacity
There are many estimates for CO2 storage capacity in the literature. The methodology employed by researchers varies considerably, so the estimates are not always directly comparable. The availability and quality of data inputs are also variable; this results in large ranges given for the estimates. For example, for US storage capacity, the DOE estimates 1877–14737 GtCO2, while the US Geological Survey (USGS) estimates 1637–4102 GtCO2.12 Even at the low end of the range, these numbers are large, representing hundreds of years of US emissions.
One way used to estimate storage capacity—termed the “volumetric method”—first estimates the total water-filled pore volume in a formation. Then, an empirical efficiency factor, generally a few percent, is applied to estimate the usable volume. The efficiency factor represents the facts that the CO2 plume will only contact a small proportion of the reservoir, and that certain parts of the reservoir may have unsuitable geologic characteristics. Multiplying the usable volume by the CO2 density in the formation yields the storage capacity.
The volumetric method assumes that storage capacity is migration limited. The migration refers to how the CO2 plume migrates through the pore space in the reservoir. However, since maintaining a safe reservoir pressure is essential, storage capacity can also be pressure limited. Many capacity estimates in the literature ignore the pressure limitations. When pressure limitations are included, they yield lower estimates than the volumetric method.13
Detailed capacity estimates have only been conducted in a few regions, such as the United States and the North Sea in Europe. Kearns et al. developed a methodology to extrapolate from these relatively known regions to give estimates for all regions worldwide.14 The basis for the extrapolation is the worldwide distribution of sedimentary basins (see figure 4). Kearns et al. estimate worldwide capacity to range from 8,000–55,000 GtCO2. Even at the lower estimate, which does take into consideration pressure limitations, there are over two hundred years of storage at current worldwide emissions rates that are approaching 40 GtCO2/year. The estimates are also comparable to the estimated 15,000 GtCO2 contained in the recoverable fossil fuels (see figure 2). While the total amount of storage space appears more than adequate, its distribution across the globe is somewhat problematic (see figure 4). North America has plentiful and well-distributed storage options, but Japan and Korea have very little. China appears to have reasonable capacities, but India has significant limitations.
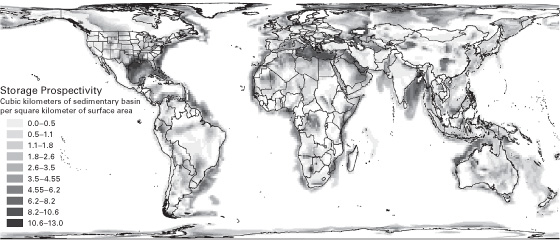
Figure 4 Geographical distribution of sedimentary basins and their thicknesses (courtesy of Jordan Kearns).
Source: Jordan Kearns et al., “Developing a Consistent Database for Regional Geologic CO2 Storage Capacity Worldwide,” Energy Procedia 114 (July 2017): 4705.
One other consideration when discussing storage capacity relates to public acceptance. NIMBY (“Not In My Backyard”) sentiments can be strong. While the world shares the benefits of CO2 storage, the abutters assume the risks. As an analogue, look at hydraulic fracturing for oil and gas production. States like Pennsylvania and North Dakota have embraced it, while states like New York have put a moratorium on it. In Europe, for the moment, offshore geologic storage projects within porous and permeable sedimentary rocks under the sea floor are operating, but onshore projects have run into resistance. Like all emerging technologies, public outreach and acceptance will be critical to its future.15