Amino Acid and Bioamine Separations
Y. Miyoshi, T. Oyama, R. Koga and K. Hamase, Graduate School of Pharmaceutical Sciences, Kyushu University, Fukuoka, Japan
Outline
6.1. Introduction
6.2. Direct Separation of Amino Acids and Amines
6.2.1. Postcolumn Colorimetric Derivatization with Ninhydrin
6.2.2. Postcolumn Fluorescence Derivatization with o-Phthalaldehyde
6.2.3. Postcolumn Fluorescence Derivatization with Fluorescamine
6.2.4. ESI–MS/MS Determination of Underivatized Amino Acids
6.3. Indirect Separation of Amino Acids and Amines
6.3.1. Derivatization with UV–Vis Reagents
6.3.2. Derivatization with Fluorescent Reagents
6.3.3. Derivatization for Mass Spectrometric Detection
6.4. Enantioselective Liquid Chromatographic Analysis of Amino Acids
6.4.1. 1-Fluoro-2,4-Dinitrophenyl-5-L-Alanine Amide _(Marfey’s Reagent)
6.4.2. o-Phthalaldehyde plus Chiral Thiols
6.4.3. (+)-1-(9-Fluorenyl)Ethyl Chloroformate
6.4.4. Cyclodextrin-Bonded Chiral Stationary Phase
6.4.5. Cinchona-Alkaloid-Bonded Chiral Stationary Phase
6.4.6. Two-Dimensional Liquid Chromatographic _Analysis of Amino Acid Enantiomers
6.5. Conclusions
6.1 Introduction
Amino acids and bioamines play crucial roles in living systems, and liquid chromatographic separation–determination techniques for amino acids and bioamines are essential tools for a wide range of research areas, including life science, food chemistry, environmental analysis, and pharmaceutical science. Liquid chromatographic methods for the determination of amino acids and amino compounds are mainly classified into two approaches. The direct separation of amino acids and amines followed by postcolumn derivatization with colorogenic or fluorogenic reagents or direct separation followed by mass spectrometric detection. The second approach is the indirect separation of amino acids and amines following precolumn derivatization with reagents suitable for the detection of these compounds with various detectors. In this chapter, we provide an overview of the liquid chromatographic analysis of amino acids and biological amines, including enantioselective analysis of amino acids.
6.2 Direct Separation of Amino Acids and Amines
Most amino acids and amino compounds do not have strong UV–Vis absorbing groups nor fluorescent moieties, therefore, derivatization processes are useful for the sensitive analysis of these compounds. The direct separation of these compounds by liquid chromatography using a postcolumn derivatization system is a simple approach, because tedious precolumn derivatization steps are not needed and most of the postcolumn derivatization methods are easily automated. For the liquid chromatographic separation of amino acids and amines, cation-exchange columns are usually employed, and ninhydrin, o-phthalaldehyde, and fluorescamine are widely used as postcolumn derivatization reagents. Sensitive LC–MS/MS methods for the detection of native amino acids have also been reported.
6.2.1 Postcolumn Colorimetric Derivatization with Ninhydrin
Ninhydrin (2,2-dihydroxyindane-1,3-dione) reacts with primary and secondary amino compounds to form characteristic colored compounds. Since its discovery by Ruhemann in 1910 [1], this colorimetric reaction has been widely used for the detection of amino acids, peptides, proteins, and amines. By the reaction with primary amino acids, a typical blue-violet-colored compound (Ruhemann purple, Figure 6.1) is formed [2,3], and a yellow-colored compound by the reaction with proline and hydroxyproline. Normally, the amino acids are separated by the cation-exchange column, and the column effluent is mixed with ninhydrin reagent. The mixture is then pumped through a reaction coil set in a boiling-water bath, and the blue-violet color is monitored at 570 nm. For the determination of proline and hydroxyproline, the yellow color is monitored at 440 nm. Fully automated LC systems originally established by Spackman, Stela, and Moore in 1958 [4] have been used with various improvements in the natural sciences for the determination of amino acids. Because the ninhydrin postcolumn-labeling LC method is reproducible and accurate, it is still one of the most useful techniques for the determination of amino acids.
6.2.2 Postcolumn Fluorescence Derivatization with o-Phthalaldehyde
A highly sensitive fluorescence reaction of amino acids was identified by Roth in 1971 [5]. After trials using o-diacetylbenzene with reducing agents, o-phthalaldehyde (OPA) was found to be a suitable fluorogenic reagent for the sensitive determination of primary amines and amino acids. OPA reacts with most of the amino acids (except cysteine, proline, and hydroxyproline) under alkaline conditions in the presence of reducing reagents to generate a bright blue fluorescence. As reducing reagents, alkylthiols, such as 2-mercaptoethanol, are widely used. The structure of the fluorescent adduct formed by the reaction of OPA and thiols with amines was determined by Simons and Johnson in 1976 [6] as 1-alkylthio-2-alkyl-substituted isoindoles (Figure 6.1). Because OPA, and the reducing reagents, alkylthiols, do not fluoresce, the OPA reaction could be used as a postcolumn fluorescence derivatization procedure for amino compounds [7]. In the OPA postcolumn derivatization procedure, amino acids are separated by a cation-exchange column similar to the ninhydrin method, and the column effluent is then mixed with the OPA reagent. Because the reaction of OPA with primary amino compounds is sufficiently rapid (within a few minutes) even at room temperature, the heating system essential for the ninhydrin reaction is unnecessary. After the reaction, the blue fluorescent derivatives are determined at 440–460 nm with excitation at 340 nm. The OPA method is 5–10 times more sensitive than the ninhydrin and fluorescamine methods.
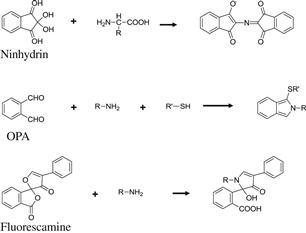
FIGURE 6.1 Reaction of postcolumn derivatization reagents with amino acids.
6.2.3 Postcolumn Fluorescence Derivatization with Fluorescamine
Fluorescamine was developed by Weigele et al. in 1972 [8], based on the fact that strongly fluorescent pyrrolinones were formed by the reaction of ninhydrin, phenylacetaldehyde, and primary amines. The reagent, 4-phenylspiro[furan-2(3H),1’-phthalan]-3,3’-dione (fluorescamine), is nonfluorescent, and it reacts with primary amines, amino acids, and peptides under aqueous conditions in a few minutes at room temperature to form intensely fluorescent substances (Figure 6.1). On the other hand, nonfluorescent derivatives are formed by the reaction of fluorescamine and secondary amino compounds. Therefore, fluorescamine can be used for the selective determination of primary amino compounds, and the fluorophore produced by the reaction is the expected pyrrolinone. Because the reaction is sufficiently rapid and the hydrolysis products are nonfluorescent, the fluorescamine reaction is applicable for the postcolumn fluorescence derivatization of primary amino compounds [9]. The amino acids are separated by a cation-exchange column similar to the ninhydrin method, and the column effluent is mixed with an alkaline-buffered solution and fluorescamine reagent. The fluorescent derivatives are detected at 480 nm with excitation at 390 nm.
6.2.4 ESI–MS/MS Determination of Underivatized Amino Acids
LC–MS/MS methods are powerful tools for the determination of various compounds in complex biological matrices. For the determination of amino acids by MS/MS detection, a volatile mobile phase is required, and therefore, an ion-pairing reversed-phase LC system with a volatile acid modifier is generally used. As an example, 76 amino acids of biological interest, including all 20 proteinogenic amino acids, can be analyzed within 15 min using LC–MS/MS [10]. The separation was performed on a short C18 column packed with 3-μm particles, and tridecafluoroheptanoic acid (TDFHA) was used as the ion-pairing agent. After the separation, the underivatized amino acids were monitored by a positive-mode ESI–MS/MS detection system, and the lower limit of quantification was from 250 fmol to 50 pmol. Considering that the lower limit of quantitation using fluorescence derivatization is around 10 pmol [7–9], the LC–MS/MS system enables the rapid and simultaneous determination of a large number of amino acids with acceptable sensitivity.
6.3 Indirect Separation of Amino Acids and Amines
For the liquid chromatographic separation of amino acids and amino compounds, indirect separation techniques after precolumn labeling of the amino group are also widely used. By the precolumn derivatization approach, amino compounds are converted into structures that are suitable for separation and suitable for detection by various sensitive detectors. For the separation of the labeled amino compounds, a wide variety of separation columns, including reversed-phase, can be used. Concerning detection, UV–Vis absorbance, fluorescence, and also MS (MS/MS) detectors are widely used, depending on the properties of the derivatization reagents.
6.3.1 Derivatization with UV–Vis Reagents
Amino acids and many small biological amines usually do not have strong absorbing or fluorescent moieties, thus derivatization approaches are often used for their determination. Because UV–Vis absorption is one of the most widely used techniques in liquid chromatography, various UV–Vis pre-column derivatization reagents for amino compounds have been reported. Below, we have summarized examples of some widely used reagents.
Phenyl Isocyanate and Phenyl Isothiocyanate
Isocyanates and isothiocyanates react with primary and secondary amino compounds, and a variety of derivatization reagents have been reported. Both aliphatic and aromatic amines react with isocyanates to form N,N’-disubstituted ureas (Figure 6.2). As an example, aliphatic amines derivatized with phenyl isothiocyanate (PITC) were nicely separated by gradient elution with a water-acetonitrile mobile phase on an ODS column [11]. The derivatives were monitored by UV absorbance at 240 nm. PITC is well-known because of the pioneering wok of Edman, who established its use for peptide sequencing [12–14]. PITC is also widely used for the determination of amino acids [15]. PITC reacts with primary and secondary amino compounds, including amino acids, under alkaline conditions to form phenylthiocarbamyl (PTC) derivatives. All the PTC derivatives of 20 proteinogenic amino acids and 22 other physiological amino acids can be separated within 60 min by reversed-phase liquid chromatography and detected at 254 nm [16].
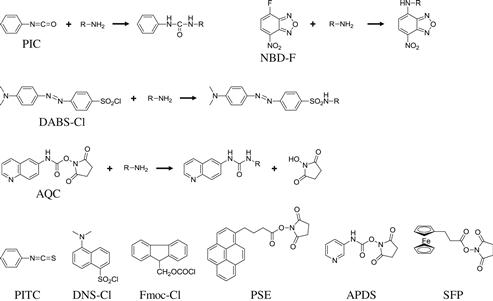
FIGURE 6.2 Reaction of precolumn derivatization reagents with amino acids.
4-N,N-Dimethylaminoazobenzene-4’-Sulfonyl Chloride
In 1975, Lin and Chang designed a sensitive chromophoric labeling reagent, 4-N,N-dimethylaminoazobenzene-4’-sulfonyl chloride (DABS-Cl), for the determination of amino acids [17]. The reagent, DABS-Cl, reacts with primary and secondary amino groups (Figure 6.2), and the dabsylated amino compounds are detected by their absorbance at around 450 nm. All proteinogenic amino acids including proline are easily derivatized within 10 min at 70°C. The resultant dabsyl derivatives of 20 proteinogenic amino acids were separated by various reversed-phase columns within about 30 min and detected simply by their absorbance in the visible region [18,19]. Because the dabsyl amino acid derivatives are stable and have sufficient sensitivity, DABS-Cl is one of the more promising reagents for the quantitative and qualitative analysis of amino compounds.
6.3.2 Derivatization with Fluorescent Reagents
Precolumn fluorescence derivatization is one of the most useful procedures for the sensitive determination of amino acids and bioamines. A large number of fluorescence labeling reagents have been reported with various combinations of fluorescent moieties and groups that are reactive with amines. The more useful ones are the fluorogenic reagents in which the reagent itself is nonfluorescent and the derivatives highly fluorescent. Because fluorescence detection is sensitive and selective by setting both the excitation and emission wavelengths, it has been widely used for the determination of trace levels of compounds in complicated biological matrices.
o-Phthalaldehyde
The OPA reagent was first reported in 1971 by Roth as a postcolumn fluorogenic reagent for amines [5] and has been widely used for the sensitive determination of primary amino compounds. However, the fluorescent derivatives are not sufficiently stable, and it is sometimes difficult to obtain reproducible results using the postcolumn derivatization system. A precolumn derivatization technique has also been developed using OPA in the presence of alkylthiol compounds such as 2-mercaptoethanol. OPA rapidly reacts with primary amino compounds within 2 min at room temperature, and the derivatives can be separated by reversed-phase liquid chromatography [20]. Fluorescence detection of the derivatives is performed at 440 nm (emission wavelength) with excitation at 330 nm. Because OPA does not react with secondary amino compounds, proline and hydroxyproline can not be determined by this method. Replacement of 2-mercaptoethanol with other thiols, such as 2-ethanethiol [21] and 3-mercaptopropionic acid [22], produced more stable fluorescent derivatives.
1-Dimethylaminonaphthalene-5-Sulfonyl Chloride
During the course of protein chemistry research looking for fluorescent compounds sensitive to the surrounding hydrophobicity, Weber designed 1-dimethylaminonaphthalene-5-sulfonyl chloride (DNS-Cl) in 1952 [23]. DNS-Cl reacts with primary and secondary amino compounds (Figure 6.2) to form a fluorescent derivative with a yellow-green fluorescence [24]. DNS–amino acids can be separated by both normal-phase and reversed-phase liquid chromatography [25] and detected by their fluorescence emission at 500–530 nm with excitation at around 350 nm. The fluorescence intensities of the DNS derivatives are stronger in organic solvents than those in the aqueous solutions frequently used for reversed-phase liquid chromatography. Nevertheless, DNS-Cl is still one of the most widely used reagents, and many applications have been reported for the determination of various amino acids and bioamines.
9-Fluorenylmethyl Chloroformate
In 1972, Carpino and Han designed a novel reagent, 9-fluorenylmethyl chloroformate (Fmoc-Cl; Figure 6.2), for the protection of the amino group during peptide synthesis [26]. Moye and Boning established, in 1979 [27], an LC method using Fmoc-Cl for the determination of primary and secondary amines, and since then, this reagent has been widely used as a fluorescent labeling reagent for amino compounds [28]. Fmoc-Cl rapidly reacts with amino compounds (within 1 min) in alkaline conditions, and the derivatives can be determined by the fluorescence emission at 310 nm with excitation at 260 nm. Because Fmoc-Cl is highly fluorescent, the excess reagent and its fluorescent hydrolysis side products should be removed prior to separation and detection [28]. The resultant amino acid derivatives can be separated by reversed-phase liquid chromatography.
6-Aminoquinolyl-N-Hydroxysuccinimidyl Carbamate
A highly reactive and sensitive derivatization reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) was reported by Cohen and Michaud in 1993 [29]. By reaction with this reagent, amino compounds are converted into urea derivatives (Figure 6.2), that can be separated by reversed-phase liquid chromatography. Fluorescence detection of AQC–amino acids is carried out at 395 nm with excitation at 250 nm (Figure 6.3). Because the emission wavelength of the AQC–amino acid is different from that of 6-aminoquinolone, a major by-product of the derivatization reaction, selective determination of amino compounds can be performed without removing excess reagent. Furthermore, the derivatization reagent AQC is immediately hydrolyzed to 6-aminoquinolone, N-hydroxysuccinimide and carbon dioxide. Therefore, the reaction mixture could be directly injected into the LC system without quenching the reaction.
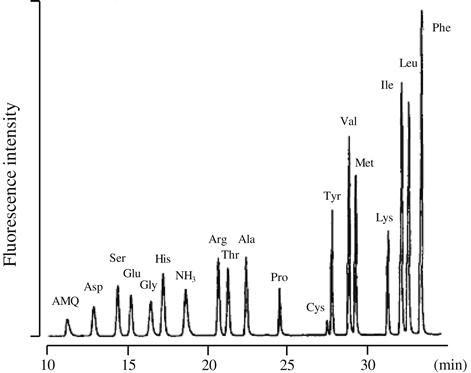
FIGURE 6.3 Reversed-phase LC separation of AQC-labeled amino acids. Separation was generated on a Waters Nova-Pak C18 column. Source: Reproduced from reference [29] with permission.
4-Fluoro-7-Nitro-2,1,3-Benzoxadiazole
During research on antileukemic compounds having the 4-nitrobenzo-2-oxa-1,3-diazole (NBD) moiety, Ghosh and Whitehouse noticed that some 7-amino derivatives of the NBD analogs were highly fluorescent. In 1968, they reported a novel fluorogenic reagent, 7-chloro-4-nitrobenzo-2-oxa-1, 3-diazole (equal to 4-chloro-7-nitro-2,1,3-benzoxadiazole (NBD-Cl)), for the determination of amino compounds [30]. As a highly reactive analog of NBD-Cl, Imai and Watanabe reported 4-fluoro-7-nitro-2,1,3-benzoxadiazole (NBD-F) in 1981 [31], which has fluorine at the p-position to the nitro group. The fluorogenic reagent NBD-F rapidly reacts with both primary and secondary amino compounds within 5 min under mild conditions (pH 7–8, 50–60°C) to produce highly fluorescent derivatives (Figure 6.2). The NBD derivatives of all proteinogenic amino acids can be separated by reversed-phase liquid chromatography, and highly sensitive detection can be carried out (except for tryptophan) at 530 nm with excitation at 470 nm [32,33].
4-(1-Pyrene)Butyric Acid N-Hydroxysuccinimide Ester (Intramolecular Excimer-Forming Fluorescence Derivatization of Polyamines)
Dipyrene derivatized molecules are known to form intramolecular excimers. The excimer exhibits a fluorescence emission at a wavelength longer than that of the monomer, and selective analysis of polypyrene-labeled compounds can be carried out using the excimer fluorescence. In 2000, Nohta et al. described a method for the determination of biologically active polyamines by intramolecular excimer-forming derivatization with 4-(1-Pyrene)butyric acid N-hydroxysuccinimide ester (PSE) [34]. By this method, dipyrene-labeled putrescine, cadaverine, spermidine, and spermine could be separated by reversed-phase liquid chromatography and specifically detected by the excimer fluorescence at 475 nm with excitation at 345 nm. The excimer fluorescence-emission wavelength is far different from that of the monomer fluorescence-emission wavelength (375 nm) derived from the excess PSE reagent, the hydrolysate product (4-(1-pyrene)butyric acid), and other monopyrene-labeled derivatives. In real biological samples, various monopyrene-labeled derivatives are formed by reaction with PSE and severely interfere with the determination of polyamines.
6.3.3 Derivatization for Mass Spectrometric Detection
Various derivatization techniques are also useful for the MS (MS/MS) detection of amino compounds. Although native amines or amino acids could be detected by MS detection, precolumn derivatization procedures are also frequently used to obtain better separation by the widely used reversed-phase columns. By using precolumn derivatization with reagents suitable for MS or MS/MS, a highly sensitive and selective determination of amino compounds can be performed.
6-Aminoquinolyl-N-Hydroxysuccinimidyl Carbamate
AQC is one of the best precolumn fluorescence derivatization reagents for amino compounds [29]. Currently, MS/MS detection is frequently used for the selective determination of biological substances in complex mixtures. The reagent AQC reacts with primary and secondary amines to form aminoquinoline-labeled compounds via a carbamide linkage. These derivatives are separated by reversed-phase liquid chromatography and can be monitored by electrospray ionization–mass spectrometry. The loss of the aminoquinoline tag occurs readily and can be monitored by MS/MS detection, thus, metabolite analysis of amino compounds can be carried out [35].
3-Aminopyridyl-N-Hydroxysuccinimidyl Carbamate
In 2009, Shimbo et al. designed a new precolumn derivatization reagent, 3-aminopyridyl-N-hydroxysuccinimidyl carbamate (APDS; Figure 6.2), for the LC–MS/MS determination of amino compounds [36]. Amino compounds easily react with APDS under mild alkaline conditions at 55°C within 10 min and can be directly injected into the reversed-phase LC system. The derivatized molecules are monitored by the common fragment ion (m/z 121) derived from the aminopyridyl moiety of the reagent. By using the rapid gradient procedure, more than 100 analytes could be selectively determined with high sensitivity within 10 min (Figure 6.4).
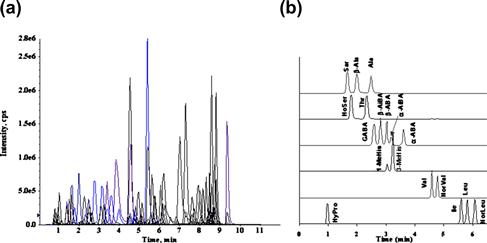
FIGURE 6.4 Overlaid mass chromatograms (a) of APDS-tagged 105 amino compounds.
An inertsil C8-3 column was used for the separation. (b) Typical chromatograms of APDS-tagged amino acids with the same mass. Source: Reproduced from reference [36] with permission.
6.4 Enantioselective Liquid Chromatographic Analysis of Amino Acids
Most amino acids have a chiral center at the alpha position, resulting in L- and D-enantiomers. High-performance liquid chromatography is the most widely used technique for the separation of amino acid enantiomers. For that purpose, various chiral derivatizing reagents and chiral stationary phases are frequently used. The following topics are typical examples of the LC separation and determination of amino acid enantiomers.
6.4.1 1-Fluoro-2,4-Dinitrophenyl-5-L-Alanine Amide (Marfey’s Reagent)
In 1984, a new reagent, 1-fluoro-2,4-dinitrophenyl-5-L-alanine amide (FDAA), was reported by Marfey for the enantiomeric separation of amino acids [37]. FDAA contains the enantiomerically pure L-alanine moiety in the reagent and reacts with amino acids to form diastereomers (Figure 6.5). As of now, many Marfey reagent analogs have been reported [38,39]. By using a reversed-phase LC system, the enantiomers of FDAA–derivatized amino acids could be separated and detected by their absorbance at 340 nm.
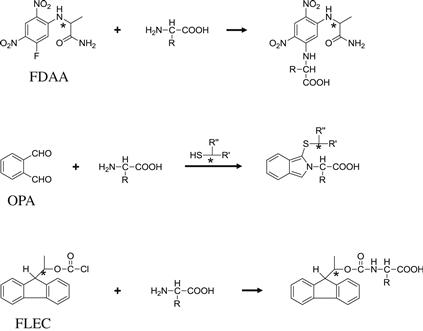
FIGURE 6.5 Reaction of chiral derivatization reagents with amino acid enantiomers.
6.4.2 o-Phthalaldehyde plus Chiral Thiols
OPA was originally established for the postcolumn derivatization of amino compounds [5], then a precolumn derivatization procedure was also reported using 2-mercaptoethanol as a thiol compound [20]. Because the fluorescent derivative is an adduct of OPA, amino acid, and thiol, the use of an optically active thiol compound results in the formation of a diastereomer (Figure 6.5). In 1984, Aswad reported a method using N-acetyl-L-cysteine [40], wherein aspartic acid enantiomers could be sufficiently separated. By a similar method, the enantiomers of 21 amino acids including 17 proteinogenic amino acids can be separated by reversed-phase liquid chromatography in about 60 min [41,42], and the derivatives were detected by their fluorescence at 443 nm with excitation at 344 nm. In 1994, Brückner et al. reported a set of chiral thiol compounds, N-isobutyryl-L-cysteine (IBLC) and N-isobutyryl-D-cysteine (IBDC), that could be utilized for the OPA procedure [43]. These thiol compounds enable complete separation of proteinogenic amino acid enantiomers in a single reversed-phase LC run within 70 min, and the elution order of the enantiomers could be reversed by the replacement of IBLC with IBDC.
6.4.3 (+)-1-(9-Fluorenyl)Ethyl Chloroformate
In 1987, Einarsson et al. designed a new chiral derivatization reagent [44], (+)-1-(9-fluorenyl)ethyl chloroformate (FLEC). This reagent is an analog of Fmoc-Cl [26], which is widely used as a fluorescence-labeling reagent for amino compounds. FLEC contains an ethyl group instead of the methyl group of Fmoc-Cl and, thus, has a chiral carbon in its structure. FLEC reacts with amino acids (Figure 6.5) within 4 min at room temperature, and the derivatives can be detected by their fluorescence at 310 nm with excitation at 260 nm. By using a reversed-phase column, the enantiomers of 17 primary amino acids could be separated within about 70 min in a single run [44].
6.4.4 Cyclodextrin-Bonded Chiral Stationary Phase
Cyclodextrins (CDs) are useful selectors for the separation of enantiomers, and various chiral stationary phases containing a CD moiety have been reported. Armstrong et al. reported the direct enantiomer separation of several amino acids using an α-CD bonded stationary phase [45]. The linkage of the α-CD bonded chiral stationary phase is hydrolytically stable, and by using the enantioselective column, 22 amino acid analogs including tryptophan, phenylalanine, and tyrosine could be separated.
6.4.5 Cinchona-Alkaloid-Bonded Chiral Stationary Phase
Cinchona alkaloids are low molecular-weight chiral selectors, and several enantioselective columns having quinine and quinidine analogs have been reported. In 2011, Reischl et al. reported an enantioselective and chemoselective method for the determination of all proteinogenic amino acids [46]. The amino acids were derivatized with succinimidyl ferrocenyl propionate (SFP; Figure 6.2) and separated by a quinine-based weak chiral anion-exchange column, QD-AX. With the mobile phase containing ammonium formate, the enantiomers of all 20 proteinogenic amino acids except the achiral glycine were separated within 10 min. Additionally, by using MS/MS detection, the chemoselective analysis of all amino acids could be performed in a single LC run.
6.4.6 Two-Dimensional Liquid Chromatographic Analysis of Amino Acid Enantiomers
For the determination of trace amounts of D-amino acids in complicated biological matrices, two-dimensional LC with two separation modes is a straightforward approach. As an example, a small amount of D-alanine (as a sensitive fluorescence derivative with NBD-F) could be determined by the combination of a reversed-phase column and a Pirkle-type enantioselective column with naphthylglycine as the chiral selector [47]. By using this system, the fraction of NBD–alanine (as the mixture of D plus L forms) was on-line collected to the loop device and automatically transferred to the enantioselective column to separate the D- and L-enantiomers. Using a microbore ODS column and a narrow bore enantioselective column, a more sensitive two-dimensional LC analysis of the NBD–amino acid enantiomers could be carried out [48]. These two-dimensional LC systems could be expanded to the simultaneous determination of multiple D-amino acids by adopting a multiloop device (Figure 6.6), and the sensitive and selective analysis of various amino acid enantiomers in real biological samples was accomplished in a single LC run [49,50].
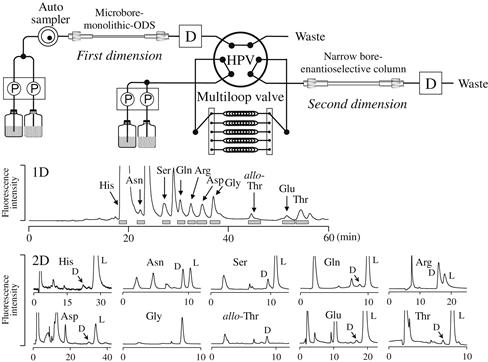
FIGURE 6.6 Flow diagram of an enantioselective two-dimensional LC system and the chromatograms obtained for rat urine.
Enantiomers of NBD–amino acids were separated on a Sumichiral OA-2500S column Source: Reproduced from reference [50] with permission.
6.5 Conclusions
Liquid chromatographic separation techniques are useful for the simultaneous determination of various amino acids and bioamines. Concerning real-world samples, uncountable numbers of interfering substances, including peptides and other amino compounds, are present in the complicated biological matrices, such as tissues and physiological fluids. Therefore, selective liquid-phase separation techniques in combination with sensitive detection systems are indispensable approaches. The development of the novel reagents as well as novel separation techniques have definitely contributed (and will also continue to contribute) to expanding the frontiers of amino acid and bioamine research.
References
1. Ruhemann S. Cyclic di- and tri-ketones. J Chem Soc. 1910;97:1438–1449.
2. Ruhemann S. Triketohydrindene hydrate, Part V The analogues of uramil and purpuric acid. J Chem Soc. 1910;97:1486–1492.
3. West R. Siegfried Ruhemann and the discovery of ninhydrin. J ChemEduc. 1965;42:386–387.
4. Spackman DH, Stein WH, Moore S. Automatic recording apparatus for use in the chromatography of amino acids. Anal Chem. 1958;30:1190–1206.
5. Roth M. Fluorescence reaction for amino acids. Anal Chem. 1971;43:880–882.
6. Simons Jr SS, Johnson DF. The structure of the fluorescent adduct formed in the reaction of o-phthalaldehyde and thiols with amines. J Am Chem Soc. 1976;98:7098–7099.
7. Benson JR, Hare PE. o-Phthalaldehyde: Fluorogenic detection of primary amines in the picomole range Comparison with fluorescamine and ninhydrin. Proc Nat Acad Sci USA. 1975;72:619–622.
8. Weigele M, Debernardo SL, Tengi JP, Leimgruber W. A novel reagent for the fluorometric assay of primary amines. J Am Chem Soc. 1972;94:5927–5928.
9. Udenfriend S, Stein S, Böhlen P, Dairman W, Leimgruber W, Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972;178:871–872.
10. Piraud M, Vianey-Saban C, Petritis K, Elfakir C, Steghens JP, Bouchu D. Ion-pairing reversed-phase liquid chromatography/electrospray ionization mass spectrometric analysis of 76 underivatized amino acids of biological interest: a new tool for the diagnosis of inherited disorders of amino acid metabolism. Rapid Comm Mass Spec. 2005;19:1587–1602.
11. Björkqvist B. Separation and determination of aliphatic and aromatic amines by high-performance liquid chromatography with ultraviolet detection. J Chromatogr. 1981;204:109–114.
12. Edman P. A method for the determination of the amino acid sequence in peptides. Arch Biochem. 1949;22:475–476.
13. Edman P. Method for determination of the amino acid sequence in peptides. Acta Chem Scand. 1950;4:283–293.
14. Edman P, Begg G. A protein sequenator. Euro J Biochem. 1967;1:80–91.
15. Heinrikson RL, Meredith SC. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984;136:65–74.
16. Cohen SA, Bidlingmeyer BA, Tarvin TL. PITC derivatives in amino acid analysis. Nature. 1986;320:769–770.
17. Lin JK, Chang JY. Chromophoric labeling of amino acids with 4-dimethylaminoazobenzene-4’-sulfonyl chloride. Anal Chem. 1975;47:1634–1638.
18. Knecht R, Chang JY. Liquid chromatographic determination of amino acids after gas-phase hydrolysis and derivatization with (dimethylamino)azobenzenesulfonyl chloride. Anal Chem. 1986;58:2375–2379.
19. Jansen EHJM, Berg RHVD, Both-Miedema R, Doorn LB. Advantages and limitations of pre-column derivatization of amino acids with dabsyl chloride. J Chromatogr. 1991;553:123–133.
20. Lindroth P, Mopper K. High performance liquid chromatographic determination of subpicomole amounts of amino acids by precolumn fluorescence derivatization with o-phthaldialdehyde. Anal Chem. 1979;51:1667–1674.
21. Fleury MO, Ashley DV. High-performance liquid chromatographic analysis of amino acids in physiological fluids: on-line precolumn derivatization with o-phthaldialdehyde. Anal Biochem. 1983;133:330–335.
22. Godel H, Graser T, Földi P, Pfaender P, Fürst P. Measurement of free amino acids in human biological fluids by high-performance liquid chromatography. J Chromatogr. 1984;297:49–61.
23. Weber G. Polarization of the fluorescence of macromolecules 2 Fluorescent conjugates of ovalbumin and bovine serum albumin. Biochem J. 1952;51:155–167.
24. Hartley BS, Massey V. The active centre of chymotrypsin 1 Labelling with a fluorescent dye. Biochim Biophys Acta. 1956;21:58–70.
25. Bayer E, Grom E, Kaltenegger B, Uhmann R. Separation of amino acids by high performance liquid chromatography. Anal Chem. 1976;48:1106–1109.
26. Carpino LA, Han GY. The 9-fluorenylmethoxycarbonyl amino-protecting group. J Organ Chemy. 1972;37:3404–3409.
27. Moye HA, Boning Jr AJ. A versatile fluorogenic labelling reagent for primary and secondary amines: 9-Fluorenylmethyl chloroformate. Anal Lett. 1979;12:25–35.
28. Einarsson S, Josefsson B, Lagerkvist S. Determination of amino acids with 9-fluorenylmethyl chloroformate and reversed-phase high-performance liquid chromatography. J Chromatogr. 1983;282:609–618.
29. Cohen SA, Michaud DP. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal Biochem. 1993;211:279–287.
30. Ghosh PB, Whitehouse MW. 7-Chloro-4-nitrobenzo-2-oxa-1,3-diazole: a new fluorigenic reagent for amino acids and other amines. Biochem J. 1968;108:155–156.
31. Imai K, Watanabe Y. Fluorimetric determination of secondary amino acids by 7-fluoro-4-nitrobenzo-2-oxa-1,3-diazole. Anal Chim Acta. 1981;130:377–383.
32. Watanabe Y, Imai K. High-performance liquid chromatography and sensitive detection of amino acids derivatized with 7-fluoro-4-nitrobenzo-2-oxa-1,3-diazole. Anal Biochem. 1981;116:471–472.
33. Hamase K, Homma H, Takigawa Y, Fukushima T, Santa T, Imai K. Regional distribution and postnatal changes of D-amino acids in rat brain. Biochimit Biophys Acta. 1997;1334:214–222.
34. Nohta H, Satozono H, Koiso K, Yoshida Y, Ishida J, Yamaguchi M. Highly selective fluorometric determination of polyamines based intramolecular excimer-forming derivatization with a pyrene-labeling reagent. Anal Chem. 2000;72:4199–4204.
35. Boughton BA, Callahan DL, Silva C, et al. Comprehensive profiling and quantitation of amine group containing metabolites. Anal Chem. 2011;83:7523–7530.
36. Shimbo K, Oonuki T, Yahashi A, Hirayama K, Miyano H. Precolumn derivatization reagents for high-speed analysis of amines and amino acids in biological fluid using liquid chromatography/ electrospray ionization tandem mass spectrometry. Rapid Comm Mass Spec. 2009;23:1483–1492.
37. Marfey P. Determination of D-amino acids, II Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Res Comm. 1984;49:591–596.
38. Brückner H, Gah C. High-performance liquid chromatographic separation of DL-amino acids derivatized with chiral variants of Sanger’s reagent. J Chromatogr. 1991;555:81–95.
39. Bhushan R, Brückner H. Use of Marfey’s reagent and analogs for chiral amino acid analysis: assessment and applications to natural products and biological systems. J Chromatogr B. 2011;879:3148–3161.
40. Aswad DW. Determination of D- and L-aspartate in amino acid mixtures by high-performance liquid chromatography after derivatization with a chiral adduct of o-phthaldialdehyde. Anal Biochem. 1984;137:405–409.
41. Buck RH, Krummen K. Resolution of amino acid enantiomers by high-performance liquid chromatography using automated pre-column derivatisation with a chiral reagent. J Chromatogr. 1984;315:279–285.
42. Nimura N, Kinoshita T. o-Phthalaldehyde-N-acetyl-L-cysteine as a chiral derivatization reagent for liquid chromatographic optical resolution of amino acid enantiomers and its application to conventional amino acid analysis. J Chromatogr. 1986;352:169–177.
43. Brückner H, Haasmann S, Langer M, Westhauser T, Wittner R, Godel H. Liquid chromatographic determination of D- and L-amino acids by derivatization with o-phthaldialdehyde and chiral thiols: applications with reference to bioscience. J Chromatogr A. 1994;666:259–273.
44. Einarsson S, Josefsson B, Möller P, Sanchez D. Separation of amino acid enantiomers and chiral amines using precolumn derivatization with (+)-1-(9-fluorenyl)ethyl chloroformate and reversed-phase liquid chromatography. Analy Chem. 1987;59:1191–1195.
45. Armstrong DW, Yang X, Han SM, Menges RA. Direct liquid chromatographic separation of racemates with an α-cyclodextrin bonded phase. Anal Chem. 1987;59:2594–2596.
46. Reischl RJ, Hartmanova L, Carrozzo M, Huszar M, Frühauf P, Lindner W. Chemoselective and enantioselective analysis of proteinogenic amino acids utilizing N-derivatization and 1-D enantioselective anion-exchange chromatography in combination with tandem mass spectrometric detection. J Chromatogr A. 2011;1218:8379–8387.
47. Morikawa A, Hamase K, Zaitsu K. Determination of D-alanine in the rat central nervous system and periphery using column-switching high-performance liquid chromatography. Anal Biochem. 2003;312:66–72.
48. Miyoshi Y, Hamase K, Tojo Y, Mita M, Konno R, Zaitsu K. Determination of D-serine and D-alanine in the tissues and physiological fluids of mice with various D-amino-acid oxidase activities using two-dimensional high-performance liquid chromatography with fluorescence detection. J Chromatogr B. 2009;877:2506–2512.
49. Hamase K, Morikawa A, Ohgusu T, Lindner aitsu WK, aitsu K. Comprehensive analysis of branched aliphatic D-amino acids in mammals using integrated multi-loop two-dimensional column-switching high-performance liquid chromatographic system combining reversed-phase and enantioselective columns. J Chromatogr A. 2007;1143:105–111.
50. Hamase K, Miyoshi Y, Ueno K, et al. Simultaneous determination of hydrophilic amino acid enantiomers in mammalian tissues and physiological fluids applying a fully automated micro-two-dimensional high-performance liquid chromatographic concept. J Chromatogr A. 2010;1217:1056–1062.