Compositional Analysis of Foods
M. Herrero, M. Castro-Puyana, E. Ibáñez and A. Cifuentes, Laboratory of Foodomics, Institute of Food Science Research (CIAL-CSIC), Madrid, Spain
Outline
11.1. Introduction
11.2. Carbohydrates
11.3. Vitamins
11.3.1. Fat-Soluble Vitamins
11.3.2. Water-Soluble Vitamins
11.3.3. Multivitamin Methods
11.4. Amino Acids, Peptides, and Proteins
11.5. Lipids
11.6. Minor Components of Food
11.6.1. Phenolic Compounds
11.6.2. Carotenoids
11.6.3. Others
11.7. Food Additives
11.7.1. Food Preservatives
11.7.2. Antioxidants
11.7.3. Colorants
11.7.4. Sweeteners
11.8. Conclusions and Future Trends
11.1 Introduction
Liquid chromatography (LC) has become a useful tool in food analysis to ascertain food quality, to implement regulatory enforcement, or to comply with national and international food standards. LC is chosen for multiple applications because it can be applied to a wide range of different samples, allows separation of compounds ranging from ions to biopolymers, and employs mild separation conditions, such as low temperature, thus precluding the thermal decomposition of unstable compounds, such as vitamins or polyphenols. LC also provides good sensitivity, resolution, and selectivity.
LC is used in food analysis for measuring major food components, such as carbohydrates, proteins, and lipids (such as triglycerides) but also minor components such as vitamins, artificial additives (sweeteners, antioxidants, colorants, preservatives, etc.), mycotoxins, amino acids, peptides, pigments, carotenoids, and polyphenols, among others. Some of these applications are discussed in this chapter. Even considering the broad range of applications observed for LC, other analytical techniques, such as gas chromatography (GC) and capillary electrophoresis (CE), occupy an important niche in the compositional analysis of food; for instance, GC is the technique of choice for aromas and other volatile compounds and, for these type of compounds, provides higher efficiencies and faster separations. On the other hand, CE is especially important when working with compounds bearing ionic charge in solution, since its separation principle is based on their relative migration in an electric field. Table 11.1 provides an overview of the different compounds that can be found in foods, together with the technique of choice in each particular case.
TABLE 11.1
Compositional Analysis of Foods and the Preferred Analytical Technique to Analyze Each Group of Compounds
| Food additives | Acidulants Antioxidants Preservatives Artificial sweeteners Colorants Flavors |
LC GC |
| Food residues and contaminants | Residues of chemotherapeutics Antiparasitic drugs Mycotoxins Pesticides |
LC GC |
| Lipids | Triglycerides Hydroperoxides Fatty acids |
LC GC |
| Carbohydrates | LC | |
| Vitamins | Fat-soluble vitamins Water-soluble vitamins |
LC |
| Amino acids | LC CE | |
| Peptides | LC CE | |
| Proteins | LC CE |
Foods are complex matrices, and frequently, the components of interest are present in small amounts compared to macronutrients, such as proteins, carbohydrates, or lipids; these compounds, when present in large amounts, can interfere with the determination of minor components. There are several ways to overcome this problem in LC: (a) to increase the specificity of the separation/detection method, (b) to improve the selectivity of the sample preparation step prior to LC, or (c) both.
Among the sample preparation techniques prior to LC, solid-phase extraction (SPE) has been widely used to remove interferences from the food matrix and to concentrate the analytes of interest. SPE is based on the selective retention of compounds on a sorbent housed in a disposable extraction minicolumn (or cartridge). To cover a wide range of polarities, several sorbents, of polar, nonpolar, ionic, and polymeric materials, can be used.
Given the concerns about the use of toxic organic solvents in food chemistry, many new techniques have been developed to overcome or minimize this problem. For instance, environmentally clean extraction techniques, such as those based on the use of compressed fluids (pressurized liquids, PLE; supercritical fluids, SFE; and subcritical water, SWE or PHWE), are widely used as alternatives to conventional procedures, such as solid–liquid extraction (SLE), liquid–liquid extraction (LLE), and the like. These alternative processes have in common the use of lower amount of solvents (from hundred milliliters to few milliliters), the lack of toxic residues, higher efficiency extraction (in terms of yields and energy used), and the improved selectivity of the process. SFE has been used in food analysis as a sample preparation technique, mainly for lipophilic compounds, while PLE has been extensively used for many compositional food applications, because the selectivity of this technique can be tailored by the solvent and temperature used during the extraction process.
11.2 Carbohydrates
Carbohydrates are one of the most important components of food and can be roughly classified depending on their molecular weight as sugars (mono- and disaccharides, low molecular weight), oligosaccharides (intermediate molecular weight), and polysaccharides (high molecular weight), which might be composed of many units of monosaccharides. Carbohydrates are the main source of energy for almost all physiological functions, including brain functions. In addition, carbohydrates exert other important effects, being extremely important for satiety, blood glucose level, insulin metabolism, and serum cholesterol as well as for colonic microflora and gastrointestinal processes, such as laxation and fermentation [1]. Carbohydrates are synthesized by all green plants, and, in the body, are either metabolized immediately or stored in the form of glycogen. Nevertheless, the body can also form some of these compounds from amino acids and the glycerol contained in fats.
Sugars (glucose, sucrose, fructose, lactose, and maltose), sugar polyols (sorbitol and mannitol), oligosaccharides (GOS, galactooligosaccharides, and FOS, fructooligosaccharides), and polysaccharides (starch and nonstarch polysaccharides) are widely regarded as the major classes of carbohydrates relevant for human nutrition. From a nutritional point of view, carbohydrates can be grouped into compounds directly available or easily metabolized, such as mono-, di-, or polysaccharides (including glucose, lactose, dextrins, and starch, among others) and unavailable compounds that cannot be directly utilized by the human organism. In this second group, structural plant polysaccharides like cellulose, pectins, and β-glucans are included [2]. Thus, the carbohydrates included in the first group are hydrolyzed by the human gastrointestinal system enzymes whereas those included in the second group are not hydrolyzed by the endogenous human enzymes. However, these latter compounds can be employed and fermented to a particular extent by the microorganisms present in the large intestine then absorbed [3]. In addition, the fermentable short-chain carbohydrates like mono-, di-, oligosaccharides, and polyols that are poorly absorbed in the small intestine might provide different effects on the gastrointestinal health. More in-depth information about the chemistry of carbohydrates can be found elsewhere [2].
Major food sources of carbohydrates include cereals, vegetables, fruits, potatoes, legumes, and flour-related products. Consequently, naturally occurring carbohydrates are widely consumed as a part of a healthy diet, although sugars can be also added to foods during manufacturing. Their analysis and determination in foods can be useful not only for nutritional purposes but also to characterize these foods in terms of quality, authenticity, flavor, maturity, or even to estimate appropriate storage conditions.
Although GC has been extensively employed for the analysis of carbohydrates (mainly sugars), their analysis by LC present a series of advantages, such as faster analysis, possibility of avoiding sample derivatization, analysis of high molecular-weight carbohydrates as well as an increase in versatility thanks to using different separation modes and detectors. Among the different separation modes able to provide good carbohydrate separations, ion-exchange chromatography, size-exclusion chromatography, and partition (reversed- or normal-phase) chromatography are the most widely employed.
Size-exclusion chromatography (SEC), based on the permeation of carbohydrates through resins of different pore size and structure, is often used to fractionate carbohydrates prior to separation or for analysis. Typically, carbohydrates with higher molecular weight elute first from the column. On the other hand, low molecular-weight carbohydrates penetrate into the three-dimensional network inside the resins, taking longer to elute. This analytical approach has been used, for instance, to determine raffinose-family oligosaccharides from lentils, as a measure of product quality [4]. These oligosaccharides may cause stomach discomfort, thus, rendering the product less valuable. Consequently, their determination in this and other vegetables is a useful means to select cultivars that produce fewer unintended side-effects.
Ion-exchange chromatography, either using anionic- or cationic-exchange resins, is effective method for carbohydrate separations, high-performance anion-exchange chromatography (HPAEC) being the method that has found greater success. Normally, highly alkaline conditions using quaternary ammonium resins are employed in order to allow carbohydrate ionization. Using this separation mode, mono- and disaccharides as well as oligosaccharides can be separated. In fact, it is possible to develop methods for the separation of interesting oligosaccharides according to their degree of polymerization. This strategy has been employed, for instance, to characterize the FOS present in fermented milks, taking into consideration their relevance in the functional food industry (see Figure 11.1) [5].
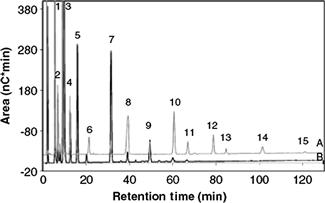
FIGURE 11.1 Chromatographic profiles of fermented milk containing added prebiotic fiber Actilight (A) and Beneo P95 (B). Peak identification: 1, glucose; 2, fructose; 3, sucrose; 4, lactose; 5, melezitose (ISTD); 6, GF2; 7, F2; 8, GF3; 9, F3; 10, GF4; 11, F4; 12, GF5; 13, F5; 14, GF6; 15, F6; 16, F7. Source: Reproduced with permission from [5]
Partition chromatography is also an alternative for the separation of carbohydrates. Hydrophilic interaction liquid chromatography (HILIC) provides improved separations compared with more-conventional reversed-phase LC methods. These HILIC-based separations are normally carried out using functionalized silica-particle columns, in which an amino group, such as a trifunctional aminopropylsilane, is bonded. This type of column was employed, for example, for the determination of several types of carbohydrates in vegetable and fruits, including oligo-, di-, and monosaccharides and polyols [1]. In the HILIC mode separations similar to those obtained in the normal-phase (NP) mode are obtained, although using reversed-phase (RP) compatible solvents for their elution. At present, new columns for HILIC that could be interesting for carbohydrates analysis are under development, like columns based on immobilized secondary and tertiary amines.
Due to their structural characteristics, carbohydrates do not possess strong UV–Vis absorption properties. So, prior to photometric detection (either DAD, UV–Vis, or fluorescence), carbohydrates have to be derivatized to increase their response. A great diversity of reagents are employed for this purpose, introducing chromophores by reaction with the hydroxyl or carbonyl groups [6]. To avoid laborious derivatization processes, other detectors might be used, such as refractive index (RI), evaporative-light scattering (ELSD), or pulsed amperometric detectors (PAD). PAD has successfully been used for carbohydrates analysis in LC because its suitability for compounds that contain electroactive functional groups and because it works better under alkaline conditions, like those usually employed in HPAEC. Nevertheless, as in other areas of LC, mass spectrometry (MS) detectors are being increasingly employed for carbohydrate detection. However, the use of MS is not free from difficulties. In fact, the typical alkaline conditions employed in HPAEC impair both the volatilization and ionization of the compounds needed for the formation of an appropriate electrospray. Therefore, usually, a desalting device has to be used between the column and the detector. Another strategy commonly followed is the addition of lithium chloride to the eluent to increase the sensitivity of the carbohydrates in the MS detector by forming lithium or chloride adducts.
11.3 Vitamins
Vitamins are essential compounds necessary for many physiological functions in the human body. Although needed in low amounts, the organism is unable to synthesize these compounds, and therefore, they have to be obtained from the diet. Consequently, their determination in foods is of great importance and covers different aspects, including food quality, labeling and regulation issues, vitamins transformation by food storage, and so forth. Vitamins are divided into two main groups depending on their solubility, fat-soluble and water-soluble vitamins.
11.3.1 Fat-Soluble Vitamins
Fat-soluble vitamins include four groups of vitamins: vitamins A, D, E, and K. The analysis of each group is usually adapted according to its particular nature.
Vitamin A has important functions in healthy vision, as well as in support of the immune system and gene expression during embryonic development. Retinol is the most common form of vitamin A in foods, although several carotenoids widely distributed in nature have a pro-vitamin A effect, β-carotene carotene being the most common. Vitamin A is mainly found in organ meats, fish oils, butter, eggs, milk, and dairy products, whereas pro-vitamin A carotenoids are found in fruits and vegetables. Vitamin A is also found as fatty acid retinyl esters. For this reason, a saponification step before LC analysis is often employed to release the bound retinol. Also, the saponification may act as a cleanup step, facilitating the subsequent analysis. Although NP separations can be used RP separations are more common; above all, if a separation of the different retinyl esters and retinol is intended. The NP is usually preferred for the adequate separation of retinol isomers. In both cases, UV–Vis detection is the most frequently used, at 325 nm.
Vitamin D is another fat-soluble vitamin that does not totally conform to the traditional definition of a vitamin, because the body is able to synthesize this compound. Two compounds are involved, ergocalciferol (vitamin D2) derived from the plant steroid ergosterol and cholecalciferol (vitamin D3), which is formed in the skin in response to sunlight. The main activity of vitamin D in the body is related to the maintenance of calcium homeostasis and exhibits antirachitic activity. In foods, vitamin D is not present in a great amount, the main sources of this vitamin being fish liver oils, some saltwater fish species (mainly, salmon or sardine), and eggs, beef, and milk. Vitamins D2 and D3 as well as their 25 hydroxyvitamin metabolites are separated using reversed-phase LC with UV detection at 265 nm. Before analysis in foods, samples are usually saponified and purified by solid-phase extraction.
Vitamin K refers to 2-methyl-1,4-naphtoquinone and all derivatives from this compound that presents an antihemorrhagic activity in animals, such as phylloquinone (vitamin K1) or menaquinone-4 (Vitamin K2). Main sources of vitamin K are some legumes, vegetable oils, and leafy green vegetables. Separations can be achieved using either normal-phase or reversed-phase chromatography with detection by postcolumn fluorescence derivatization. Alternatively, UV–Vis detection can be employed at 245 nm. It is also usual, when analyzing these compounds, to introduce sample cleanup or preconcentration steps based on SPE.
The vitamin E family comprises different compounds: namely, the four tocopherols and their corresponding equivalent tocotrienols. Vitamin E is a good antioxidant that has an important function in the body for the prevention of free radical damage, although other important functions are also attributed to these components. This vitamin is common in foods, although probably, vegetable oils and some nuts are the main sources for α-tocopherol. As for other fat-soluble vitamins, the different forms of vitamin E are usually analyzed and separated using NP or RPLC. Although the more-extended C18 columns are often employed for the separation of these compounds, NP-based separations are also widely employed, mainly, because of the higher selectivity for the separation of β and γ isomers. Detection is usually by fluorescence (290 nm ex, 330 nm em.). Nevertheless, owing to its higher availability, photodiode-array detector (DAD) or UV–Vis detectors are also widely employed. The most frequent wavelength for their detection is 292 nm. Recently, MS detection was also employed. As for other, relatively low-polarity components, atmospheric pressure chemical ionization is more favorable than electrospray for their ionization.
The simultaneous determination of fat-soluble vitamins is possible, thus, simultaneous analysis of retinol, tocopherols, as well as other carotenoids present in milk, among other products, can be achieved by ultra-performance liquid chromatography (UPLC) with detection at 325, 292, and 450 nm, respectively. Another possibility is to select a compromise wavelength, in which the overall sensitivity is acceptable. For instance, 265 nm can be employed for the detection of vitamins E and D in foods using an RP method. In addition, the sample preparation method has to be carefully selected, given that several compounds are involved. In general, compromise conditions should be selected to have a maximum recovery for all of the vitamins. Saponification is one of the most-common sample treatments for the subsequent simultaneous analysis of fat-soluble vitamins.
11.3.2 Water-Soluble Vitamins
Water-soluble vitamins are formed by a wider group of compounds with different functions in humans. In fact, the number of water-soluble vitamins is higher than for the fat-soluble vitamins. The analysis of these compounds by LC usually implies different extraction–sample treatment steps due to the possibility of finding these vitamins in foods bound to other components. In addition, the chemical nature of most water-soluble vitamins impairs their analysis because of the similarity with other components that may be coextracted and analyzed.
Vitamin C, L-ascorbic acid, is one of the most important water-soluble vitamins. The main function of this compound is to prevent and treat scurvy and to act as an antioxidant, although it is also related to other functions. As a result of this activity, this compound is regarded as a potential protective agent against cancer and atherosclerosis, among other diseases. In foods, L-ascorbic acid is widely distributed, above all, in fruits and vegetables. This compound is very prone to degradation or oxidation. This is one of the major problems when analyzing vitamin C by LC. For this reason, acids are frequently employed, in both the sample preparation phase and the LC mobile phases chosen for the separation to minimize vitamin C degradation. RPLC is the method of choice vitamin C analysis with either UV or MS detection. The MS detector provides better specificity, considering that the UV–Vis absorption of L-ascorbic acid is quite similar to other natural compounds frequently found in food making its identification difficult.
Vitamin B1 (thiamin) is related to beriberi, a disease associated with a deficiency of this vitamin. In fact, thiamin is a coenzyme in different biochemical reactions. Pork, legumes, as well as liver and kidney products are regarded as excellent sources of this vitamin. Thiamin, as well as other water-soluble vitamins, is frequently found bound to proteins or carbohydrates or even phosphorylated. Therefore, prior to their analysis, a sample treatment to release the free forms of the vitamin is common. A typical extraction protocol for water-soluble vitamins includes autoclaving the sample with hydrochloric acid for the acid hydrolysis of the vitamin followed by an adjustment in the pH to values around 4.0–4.5, adequate for an enzymatic treatment. This vitamin can be, subsequently, separated by ion-pair RP chromatography and detected with a fluorescence detector after postcolumn oxidation to thiochrome. MS detection through electrospray ionization is also used, although the separation pH should be adjusted to maximize the ionization of the vitamin.
Riboflavin (vitamin B2) also acts as a cofactor and is a precursor for the coenzymes flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). These coenzymes are used in metabolism and catalyze numerous oxidation–reduction reactions. Among the good dietary sources for riboflavin, most animal-derived products, milk and dairy products, are pointed out. Foods are usually pretreated before analysis of riboflavin following similar procedures to those described for vitamin B1. Similarly, fluorescence detection is mostly employed (370 nm ex., 520 nm em.) after RP separation.
Pantothenic acid, also known as vitamin B5, is widely distributed in food, since it is a component in the coenzyme A structure. Therefore, it is essential to all organisms and its deficiency is uncommon. In addition, being part of this coenzyme, for the total vitamin B5 determination, an enzyme hydrolysis is necessary prior to analysis. Foods richest in pantothenic acid are organ meats, egg yolk, and whole grains. RP separations are employed to analyze pantothenic acid, which does not possess any specific UV–Vis absorption. To overcome this problem, either fluorescence detection or MS detection is employed.
In contrast to pantothenic acid, folic acid (vitamin B9) deficiency is more common. Normally, under the term vitamin B9 a series of compounds formed by the same folic-acid structure, differing in the number of glutamates residues attached to their structure. These molecules are used as cofactors and serve as acceptors and donors of one-carbon unit in a variety of reactions involved in amino acid and nucleotide metabolism. Although folates are present in most foods, legumes, leafy green vegetables, citrus and other fruits, and liver are regarded as good sources. The determination of folates is often performed by RPLC. If total folate is to be determined, enzymatic hydrolysis is needed to deconjugate all the forms to the corresponding monoglutamates. In addition, given the complexity of most food samples, an SPE cleanup protocol is often required. UV–Vis detection (at 290 nm) is suitable for samples with a high vitamin concentration. If not, fluorescence detection has to be used to enhance sensitivity.
Niacin refers to a group of compounds also known as vitamin B3, presenting similar biological activity, including nicotinamide, nicotinic acid, as well as other pyridine nucleotide structures. In the body, these compounds act as cofactors in oxidation–reduction reactions. To determine the total vitamin B3 content, either an acid or alkaline hydrolysis is necessary. The separation is normally performed by RPLC with fluorescence (322 nm ex., 380 nm em.) or UV detection (254 nm).
Although pyridoxine, pyridoxamine, and pyridoxal are different forms of vitamin B6, pyridoxal-5’-phosphate is the metabolically active form, acting as a cofactor in different reactions involving amino acids, mainly, transamination. Good food sources of this vitamin include meat, poultry, fruit, potatoes, and some vegetables. From the different forms in which vitamin B6 is found in food, phosphorylated pyridoxal is generally the most frequent. Therefore, acid hydrolysis followed by enzymatic hydrolysis is the most common sample preparation procedure before RPLC with a buffered, low-pH mobile phase and UV or fluorescence detection.
Vitamin B12 comprises a family of compounds generically called cobalamin. This coenzyme is particularly rich in animal products, such as meat, seafood, eggs, and milk. Therefore, as cobalamin is normally found with proteins, different steps for its purification have to be carried out before chromatographic analysis. Among them, enzymatic hydrolysis is frequently used, together with a purification protocol using SPE. The LC analysis is usually carried out by RPLC, with water, methanol, or acetonitrile as typical solvents. The UV detection of these components is also possible.
There is an obvious interest in the development of analytical methods able to determine multiple vitamins simultaneously. Nevertheless, as it has been described in this section, each water-soluble vitamin has different optimum conditions for extraction and analysis. Therefore, the most commonly employed strategy is the development of protocols for different groups of vitamins that can be simultaneously and properly analyzed under the same analytical conditions, grouping vitamins that can be treated and extracted under the same conditions. For instance, the combination of a RP separation coupled to different detectors, namely, UV, fluorescence, and MS, allows the simultaneous determination of vitamins B1, B2, B3, B6, B9, pantothenic acid, biotin, and vitamin C [7].
11.3.3 Multivitamin Methods
The simultaneous determination of fat- and water-soluble vitamins is not straightforward. On the one hand, the extraction of the vitamins from the food sample may not be adequate for all the studied compounds, considering the differences among the polarities of the different vitamins. In addition, from the analytical side, although RP conditions could theoretically allow the separation of all these compounds, the separation time would be too high or the resolution too low. One strategy is to process the sample sequentially to extract in different steps fat-soluble and water-soluble vitamins and to proceed with their analysis separately. Nevertheless, we already described a screening method based on the use of pressurized-liquid extraction and LC–DAD to simultaneously extract and analyze ascorbic acid, thiamin, nicotinamide, β-carotene (pro-vitamin A), and tocopherol acetate, among other compounds [8], using different wavelength detections. However, it is important to keep in mind that the exhaustive extraction of all the vitamins and vitamers related to them from a food sample would require laborious protocols, including hydrolysis, enzymatic treatments, and purification steps, depending on the particular vitamin, thus making impossible the simultaneous exhaustive determination of all the components.
MS/MS detection was shown to be a useful tool for the simultaneous determination of vitamins using stable isotope-labeled internal standards for their quantification in nutritional formulations [9]. Nevertheless, it is difficult to optimize the ionization and detection conditions, due to the different nature of the vitamins analyzed. Thus, frequently, compromise conditions have to be selected. Another critical factor for the multiple separation and detection of vitamins is the pH. In general, low pH values should be used, although the best conditions should be optimized in each case.
11.4 Amino Acids, Peptides, and Proteins
Amino acids, peptides, and proteins play important roles in determining the quality, nutritional, and functional properties of food products. Hence, there is a high interest in their analysis.
While free amino acids can be directly extracted from food matrices, a preliminary hydrolysis step (normally microwave-assisted to speed up the hydrolysis reaction) is necessary to liberate protein-bound amino acids. After that, a sample cleanup using a precipitation agent, ultrafiltration, or SPE on C18 cartridges is carried out to remove interferences and concentrate these compounds.
Regarding peptide analysis, ultrafiltration is usually applied to remove proteins and to fractionate peptides. SPE with C18 cartridges is also a common procedure, since it facilitates peptide fractionation together with enrichment and purification. Regarding sample preparation of proteins, they can be isolated and fractionated in different classes by selective precipitation, which involves procedures such as dialysis, ultracentrifugation, SPE, and selective precipitation using organic solvents or salts. In addition, protein digestion using endoproteases (e.g., trypsin) can be applied to carry out the MS analysis of the resulting peptides.
Ion-exchange chromatography (IEC) followed by postcolumn derivatization with derivatizing agents such as ninhydrin, fluorescamine, or o-phthalaldehyde (OPA), among others, for many years has been the most popular methods for the analysis of amino acids [10]. In recent years, RP LC with C8 or C18 columns is widely used for the separation of amino acids after precolumn derivatization (with OPA, FMOC, DNS-Cl, phenylisothiocyanate [PITC], or 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate [AQC]) with mobile phases composed of a mixture of an organic solvent (acetonitrile, methanol, or tetrahydrofuran) and acetate or phosphate buffer. Fluorescence or UV–Vis detectors are used depending on the derivatizing agent employed.
Peptide separation are possible using SEC, IEC, or RPLC (and even combinations of them). However, RPLC with silica-based columns is the most frequently employed, although polymeric sorbents and composite materials are also used as stationary phases. Mixtures of water with acetonitrile, methanol, or isopropanol containing trifluoroacetic acid (due to its ion-pairing capacity) are usually used as the mobile phase. Prior to LC analysis, derivatization of peptides is often required to improve sensitivity, although peptides with aromatic amino acids may be detected by UV or fluorescence without a derivatization step. In recent years, the coupling LC–ESI–MS and the use off-line matrix-assisted laser desorption–time-of flight (MALDI–TOF) MS have enabled a significant advance in the direct characterization and sequencing of food peptides to be achieved [11,12].
Protein analysis can be performed by RPLC, hydrophobic-interaction chromatography (HIC), IEC, SEC. Again, RPLC is by far the most widely used. The main drawback with conventional phases for analyzing proteins is slow mass transport, which results in poor resolution and long separation times [13]. For fast separations with acceptable resolution, different chromatographic phases, such as pellicular packings, perfusion packings, and monolithic columns, has been demonstrated. Most RPLC separations of proteins are carried out using gradient elution with water: ACN containing trifluoroacetic as the ion-pairing agent. The common detectors employed are UV and MS. UV is useful to compare protein profiles, whereas MS is required for protein identification. Nowadays the on-line coupling LC–MS is widely used for the analysis of allergenic proteins, detection of protein modifications, the identification of protein genetic variants, and for information on protein interactions [11,12].
Multidimensional liquid chromatographic methodologies are also used to identify peptides and proteins. In most of these applications, the first dimension is used to isolate fractions, which are analyzed in the second dimension by LC–MS [14,15].
11.5 Lipids
Lipids are present as ingredients in most foods and play key nutritional roles, being crucial for many physiological functions, they also can be used as food quality markers. For these reasons, the characterization of the lipid fraction present in fats and oils is important for the food industry. Following a general lipid classification, triacylglycerols (mono- and di- derivatives), phospholipids, glycolipids, waxes, and sterol esters are included in the saponifiable fraction, whereas the nonsaponifiable fraction comprises mainly free fatty acids, steroids, carotenoids, monoterpenes, and tocopherols.
The characterization of food lipids has historically been performed by GC, however, nowadays, there is no doubt that LC has become one of the separation techniques in common use for their analysis. Although almost all the lipids can be separated by LC, the main applications of this technique are focused on the determination of high-molecular-weight compounds like triglycerides and some related compounds.
Before analysis, lipids from solid foods are extracted with either a single, nonpolar solvent or a mixture of several organic solvents, such as hexane/2-propanol or chloroform/methanol, followed (if necessary) by sample cleanup procedures. In case of liquid foods (such as oils), the extraction step is often unnecessary, so that the samples can be injected directly into the LC system.
Both NPLC and RPLC have been used in lipid analysis by LC. The first mode has the capability to separate different classes of lipids based on the polar head groups in a single injection, whereas the RPLC mode is mainly used for investigating the molecular diversity within a given lipid class based on both the molecular size and the degree of unsaturation of molecules. This RPLC mode is that most commonly used for the separation of triacylglycerols. For lipid-class separations, chemically bonded phases (nitrile, diol, or polyvinylalcohol phases) produce better results than silica gel.
Silver-ion chromatography (Ag+ LC) has been successfully used for the separation of all lipid classes; for instance, it is widely employed for the separation of triacylglycerols. Here, the elution order is related to the increasing degree of unsaturation and the configuration of the double bonds within each fatty acid, for the separation of positional isomers. In addition, SEC with two columns in series has been used, for instance, to separate oxidized triacylglycerols or to quantify products of oxidation in methyl ester of several fatty acids. Since every unsaturated lipid can undergo oxidation preparative LC is useful for the isolation of oxidation products for subsequent identification.
Because many fat molecules (for example, fatty acids) do not contain a chromophore, detection by UV–Vis cannot be used. In these cases, a derivatization step is necessary to allow their detection. Other usual LC detection methods include refractive index (RI) and evaporative light scattering (ELS). RI is affected by changes in the pressure and temperature of the mobile phases, so that only isocratic elution can be performed, limiting the separative possibilities. ELS has proven useful for the NPLC analysis of lipids for quantitative application, where information on molecular diversity within a lipid class is not desired. The main disadvantage of both detection methods is that sensitivity is not satisfactory [13].
LC–MS is a powerful tool for the identification of lipids. Among the ionization methods, atmospheric pressure chemical ionization (APCI) provides the best results for the identification of triglycerides, due to the easy ionization of nonpolar triglycerides, the good compatibility with nonaqueous mobile phases, and good sensitivity. Although reports of RPLC-MS using APCI for the characterization of triacylglycerols or triacylglycerol oxidation products can be found in the literature. In addition, Ag+ LC–APCI–MS has been used for the regioisomeric analysis of triacylglycerols.
Monodimensional LC separations are often insufficient to resolve all the lipids of interest in food analysis; however, a full analysis of these samples may be attained by combining two independent separation steps with different selectivity, enhancing the resolving power of LC. Following this idea, different comprehensive two-dimensional LC approaches combined with MS have been described for the complete separation of triacylglycerols [16,17].
11.6 Minor Components of Food
11.6.1 Phenolic Compounds
Phenolic compounds comprise different families of compounds widely distributed in nature, found in most foods of vegetable origin. Their large chemical variability includes several thousands of compounds, from simple phenolic acids to complex flavonoids. These compounds have attracted much attention because of their influence on different organoleptic parameters, such as color or taste. However, at present, the interest focused on these components is even higher and relies on the beneficial health properties that they are thought to confer [18]. Phenolic compounds are usually potent antioxidants, although some of them are also regarded as antimicrobial or anticarcenogenic.
The number of articles devoted to the separation of phenolic compounds is huge. Basically, RP separations are employed, using C18 columns. The solvents most widely employed are water, methanol, and acetonitrile, frequently selecting low pH by adding an acid to improve the separation. Since some phenolic compounds are glycosylated, it is not unusual to apply an acid hydrolysis pretreatment to release the aglycones. UV detection is widely employed since the UV–Vis spectrum of the phenolic compounds can be helpful for identification purposes. Nevertheless, the use of MS is now common, as a consequence of the good ionization yield that these compounds provide when using electrospray. Considering the great variability of these compounds as well as the diversity on the chemical composition of some foods, such as beer, wine, olive oil, and fruit, different methods are being constantly developed to increase the separation power and minimize the sample treatment. Strategies to achieve this goal include the use of UPLC, new columns (such as fused-core particles), and the application of multidimensional liquid chromatography. Several developments have shown the applicability of combining two different RP columns to separate multiple phenolic compounds by comprehensive two-dimensional LC [19].
11.6.2 Carotenoids
The importance of the analysis of carotenoids in food relies on the fact that these compounds are one of the most important kinds of naturally occurring pigments. Carotenoids are highly appreciated, not only for the pro-vitamin A activity but also for the important health benefits they are claimed to possess [20]. Although LC is widely used for their analysis, carotenoid separation and identification is far from being easy, mainly due to their chemical variability. Carotenoids are usually based on C40-tetraterpenoid structures, forming a symmetrical skeleton with a centrally located, extended, conjugated double-bond system, which provides a suitable chromophore for detection. Carotenoids are mainly separated by RPLC, although NPLC is useful in some cases. RPLC has the advantage of providing better separation between isomers. In this regard, although C18 columns have been traditionally used, in recent years, C30 columns are being used to a greater extent. These columns allow the separation of carotenoid isomers as well as an improved carotene separation (usually less polar). Regarding the mobile phases employed, very low polar organic solvents, such as methyl tert-butyl ether, are used in combination with methanol or acetonitrile. Typically, almost all carotenoids in a mixture can be determined by their adsorption and one of three UV-visible wavelengths. This property allows the identification of each type of carotenoid according to its UV–Vis maxima. Nevertheless, given the great chemical variability as well as their similarity, it is often difficult to properly differentiate among compounds based only on their UV–Vis spectra. For this reason, the combined use of a DAD detector with a MS detector is very useful. In this way, it is possible to obtain a double confirmation, being possible to differentiate between compounds with similar UV–Vis spectra but different molecular weights and between compounds with similar molecular weights that can have different UV–Vis spectra. APCI interfaces operating in the positive ionization mode are most commonly employed for this purpose, considering the relative low polarity of carotenoids.
To simplify carotenoids analysis, saponification procedures are employed before LC to release all the carotenoid esters as their respective free forms. However, it has been demonstrated that this saponification may change the native carotenoid composition, producing a loss of compounds as well as isomerization or artifact formation. To avoid this problem and increase the separation power to determine the intact carotenoid composition in food samples, different strategies have been followed, such as the coupling of different C30 columns [21] or the application of comprehensive two-dimensional LC [22]. This latter technique, combined with DAD and MS detection, significantly increases the separation and identification power (Figure 11.2).
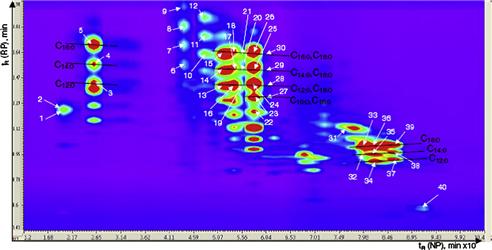
FIGURE 11.2 Two-dimensional plot (450 nm) of the comprehensive two-dimensional LC carotenoid analysis of intact red orange essential oil. Source: Reproduced with permission from [22]. Copyright 2008. American Chemical Society.
11.6.3 Others
Chlorophylls and their derivatives are found, for example, in many vegetables, and they are also used as food colorants. They have a strong hydrophobic character, so they are usually analyzed by RPLC with a C18 column and an organic mobile phase, except when chlorophyll c (polar molecule) is present. In this case, the addition of a small amount of water to the organic solvent is recommended. Spectrophotometric and MS detectors are the most commonly employed detection modes for the analysis of chlorophylls.
11.7 Food Additives
The growing concern about food safety has made the analysis of food additives and contaminants an area of great development in recent years, in which chromatographic techniques, and more specifically LC, are playing an essential role. Analysis of food additives is mandatory in processed foods that contain compounds intentionally added to improve shelf life, quality, or organoleptic characteristics, all of them should be determined to ensure compliance with national and international food standards and to fulfill legal requirements. Among the different food additives, preservatives, antioxidants, colorants, and sweeteners are discussed next.
11.7.1 Food Preservatives
Food preservatives are used primarily to prevent or retard microbial growth. The most typical food preservatives are sorbic acid, benzoic acid, propionic acid, and methyl-, ethyl- and propyl-esters of p-hydroxybenzoic acid (PHB, parabens).
Sample preparation depends mainly on the matrix; for example, liquid matrices, such as beverages, require only filtration prior to the direct injection into a LC system. For solids, steam distillation and solid–liquid extraction using solvents such as ethanol, methanol, or mixtures of acetonitrile, 2-propanol, and ethanol, are the techniques most commonly used to improve sensitivity and reduce matrix interferences. A common practice for complex samples involves precipitation of proteins and fat by the addition of methanol, followed by centrifugation or filtration, providing an extract suitable for chromatographic analysis.
Analysis by LC is usually performed by RPLC employing both isocratic or gradient elution and UV detection at specific wavelength, ranging from 230 to 275 nm. Simultaneous detection of all preservatives is a common practice in the food industry and requires the use of gradient elution and diode array or MS detection. Simultaneous quantification of 18 preservatives in beverages using time of flight LC-MS employs a C18 column (150 mm × 2.1 mm and 3.5-μm particle size), at 35°C, with a gradient of acetonitrile and water (when working in the negative-ion mode) and acetonitrile and 20 mM ammonium acetate with 0.1% formic acid (when working in the positive-ion mode). Limits of detection using this methodology ranged from 0.05 to 0.0005 mg/kg, being significantly lower than the maximum residue level established for these preservatives [23].
11.7.2 Antioxidants
Antioxidants are compounds present naturally in foods, such as tocopherols (vitamin E) or ascorbic acid (vitamin C), or formed during food processing (such as baking or smoking). A different category accounts for synthetic antioxidants intentionally added to foods to retard rancidity by preventing the oxidative degradation of lipids. Rancidity can lead to a decrease in food’s nutritive value and the production of undesirable volatiles, giving unpleasant smells and tastes. In most countries where antioxidants are permitted either singly or as combinations in foodstuffs, maximum levels for these compounds have been set.
Synthetic antioxidants that can be used in food products are the following: BHA (1(or 3)-(t-butyl)-4-hydroxy anisole), PG (propyl gallate), TBHQ (t-butyl hydroquinone), NDGA (Nor dihydroguaiaretic acid), OG (octyl gallate), DG (dodecyl gallate), and BHT (butylated hydroxytoluene), which is still allowed in some countries, although a high toxicity and mutagenicity has been demonstrated in rats. Most synthetic antioxidants are used as mixtures and at low concentrations, thus, their determination and quantification is challenging. Sample preparation usually includes extraction with organic solvents, such as acetonitrile or water-alcohol mixtures, although care must be taken in the complete determination of some antioxidants susceptible to losses due to evaporation, such as BHA, BHT, and TBHQ. The most commonly used protocol in LC is RPLC with C18, C8, or C2 stationary phases; other stationary phases and mechanisms also used include ion-exchange and chiral LC. Mobile phases in RPLC include the use of gradients of water–acetonitrile with acetic acid. UV detection at 280 nm is the most commonly used. To be able to achieve lower detection limits, specific detectors such as fluorescence, amperometric, or mass spectrometry are used.
11.7.3 Colorants
Color is a prime sensory quality by which foods are selected; color gives information about quality, sanitary status, and even texture. Colorants have been added to foods since ancient times to intensify the natural color of a food, to correct changes in color due to processing or storage, to improve aspect, or even to protect light-sensitive vitamins during food storage. Moreover, food colorants have been also used to adulterate food, artificially masking its real aspect or sanitary conditions. Food colorants can be natural or synthetic, with synthetic food colorants often selected to provide more regular dying, higher stability, and a wider variety of colors. Synthetic colors can be classified according to their chemical structure as azo (mono, di, and tris), indol, triphenylmethane, and methin dyes. In terms of safety, most synthetic colorants have been evaluated in terms of toxicity, although their status differs throughout the world.
Food dyes are extracted from foods by using adsorbents such as wool fibers, powdered polyamide, cellulose exchange resins, or RP cartridges (Sep-pak C18), which is the method of choice for a fast and easy cleanup. After desorption of the colorants and filtration, the solution is analyzed by either ion-pair or reversed-phase LC. Ion-pair LC uses water:acetone mixtures (80:20) with tetrabutylammonium chloride added as ion-pair agent and C18 or C8 columns. As for RPLC, a combination of phosphate buffer:methanol, water:acetonitrile, and methanol:acetone are commonly used. Gradients are used to allow the separation of the different classes of food dyes. Considering the strong and selective absorbance of such compounds at a particular wavelength, a UV–Vis detector is used to identify and quantify the amount of colorant added.
11.7.4 Sweeteners
Nonnutritive (low-calorie) sweeteners are widely used in foods and soft drinks. However, investigations on the toxicity of these compounds (especially saccharin, aspartame, cyclamate, and acesulfame-K) have raised questions about their safety. Their daily use is regulated through legislation, and as a result, their concentration in foods and beverages should be determined to prevent excessive intake.
Soft drinks can be analyzed by direct injection, although sometimes, clarification or degasification is needed. For most complex samples, such as jams, sweets, or desserts, sample preparation comprises homogenization, extraction, cleanup, and concentration.
Among the different techniques employed for sweetener analysis, LC is the most popular choice. LC procedures are based on isocratic or gradient RP chromatographic conditions. Detection systems used include UV, MS, fluorescence, electrochemical, and light scattering. Although some methods have been developed to simultaneously analyze different sweeteners, the common approach is to use specific methods to quantify each sweetener separately, this is mainly due to the difficulty of detecting some of the sweeteners (such as cyclamate and sucralose) using conventional UV detection and to the high sensitivity needed to detect very small amounts of such compounds in foodstuffs.
RPLC with MS detection was used for the analysis of seven artificial sweeteners (aspartame, saccharin, acesulfame-K, neotame, sucralose, cyclamate, and alitame) and one natural sweetener (stevioside). Samples were extracted using methanol:water and injected without any cleanup into the LC–MS system. Separation is carried out using a C18 column and gradient elution. Sweeteners were quantified using selective-ionization recording (SIR) at m/z 178, 397, 377, 293, 641, 312, 162, and 182 for cyclamate, sucralose, neotame, aspartame, stevioside, alitame, acesulfame-K, and saccharin, respectively, with a warfarin sodium (m/z = 307) used as an internal standard [24]. For a detailed discussion of other analytical methods to determine artificial sweeteners, refer to [25].
11.8 Conclusions and Future Trends
The application of LC techniques has allowed the determination of a large number of food compounds, including carbohydrates, vitamins, amino acids, peptides, proteins, lipids, phenols, and carotenoids as well as other minor food components and food additives. Other compounds, such as contaminants and toxins, are also typically analyzed in foods by LC methods; and they are described in a different chapter in this book. The use of LC can therefore be considered generalized in food analysis. In addition, the coupling of LC with advanced mass spectrometry detectors is making possible the determination of an even broader number of compounds with higher sensitivity, selectivity, and specificity. The development of on-line sample preparation steps in LC and LC–MS systems is also expected to become routine in food analysis.
It is also expected that applications of multidimensional LC × LC techniques in food analysis will continue to grow. These techniques provide extraordinary gains in separation power that make them ideal for the analysis of complex matrices such as foods. Although the coupling of different chromatographic separations is not new, the technological development has led, above all, to an increase of comprehensive applications, in which the whole sample is analyzed in two independent dimensions, reducing the sample preparation steps.
Nowadays, the modern trend to apply powerful foodomics-based approaches in food science [26] is also increasing, in order to obtain profiles or fingerprints of foods that can assure their traceability, authenticity, and quality as well as their effect on human health at the molecular level. In this new field, the hyphenation of LC, UPLC, or nano-LC techniques with high-accuracy and fast mass spectrometers, such as QTOF or Orbitrap. is expected to provide unbeatable results.
Acknowledgment
This work was supported by AGL2011-29857-C03-01 (Ministerio de Economía y Competitividad Spain), and CSD2007-00063 FUN-C-FOOD (Programa CONSOLIDER, Ministerio de Educacion y Ciencia, Spain). M.C.P. and M.H. thank the Ministerio de Ciencia e Innovación for their “Juan de la Cierva” and “Ramón y Cajal” contracts, respectively.
References
1. Muir JG, Rose R, Rosella O, et al. Measurement of short-chain carbohydrates in common Australian vegetables and fruits by high-performance liquid chromatography (HPLC). J Agri Food Chem. 2009;57:554–565.
2. Cui SW. Food carbohydrates: chemistry, physical properties and applications. Boca Raton, FL: CRC Press; 2005.
3. Hounsome N, Hounsome B, Tomos D, Edwards-Jones G. Plant metabolites and nutritional quality of vegetables. J Food Sci. 2008;73:R48–R65.
4. Tahir M, Vandenberg A, Chibbar RN. Influence of the environment on seed soluble carbohydrates in selected lentil cultivars. J Food Comp Anal. 2011;24:596–602. doi 10.1016/j.jfca.2010.04.007 in press.
5. Borromei C, Cavazza A, Merusi C, Corradini C. Characterization and quantitation of short-chain fructooligosaccharides and inulooligosaccharides in fermented milks by high-performance anion-exchange chromatography with pulsed amperometric detection. J Sep Sci. 2009;32:3635–3642.
6. Lamari FN, Juhn R, Karamanos NK. Derivatization of carbohydrates for chromatographic, electrophoretic and mass spectrometric structure analysis. J Chromatogr B. 2003;793:15–26.
7. Chen P, Atkinson R, Wolf WR. Single-laboratory validation of a HPLC-DAD-FD/MS method for simultaneous determination of water-soluble vitamins in multivitamin dietary tablets. AOAC Int. 2009;92:680–688.
8. Mendiola JA, Martin-Alvarez PJ, Señorans FJ, et al. Design of natural food antioxidant ingredients through a chemometric approach. J Agri Food Chem. 2010;58:787–792.
9. Phinney KW, Rimmer CA, Brown J, Sander LC, Sharpless KE, Wise SA. Isotope dilution LC-MS methods for fat- and water-soluble vitamins in nutritional formulations. Anal Chem. 2011;83:92–998.
10. Peage RW, Gilani GS. Chromatographic determination of amino acids in food. J AOAC Int. 2005;88:877–887.
11. Careri M, Mangia A. Analysis of food proteins and peptides by chromatography and mass spectrometry. J Chromatogr A. 2003;1000:609–635.
12. Mamone G, Picariello G, Caria S, Addeo F, Ferranti P. Analysis of food proteins and peptides by mass spectrometry-based techniques. J Chromatogr A. 2009;1216:7130–7142.
13. Conte LS, Moret S, Purcaro G. HPLC in food analysis. In: Corraline D, ed. Handbook of HPLC. 2nd ed Boca Raton, FL: CRC Press; 2011.
14. Herreo M, Ibáñez E, Cifuentes A, Bernal J. Multidimensional chromatography in food analysis. J Chromatogr A. 2009;1216:7110–7129.
15. Dugo P, Kumm T, Cacciola F, Dugo G, Mondello L. Multidimensional liquid chromatographic separations applied to the analysis of food samples. J Liquid Chromatogr Rel Tech. 2008;31:1758–1807.
16. Dugo P, Kumm T, Crupi ML, Cotroneo A, Mondello L. Comprehensive two-dimensional liquid chromatography combined with mass spectrometry detection in the analyses of triacylglycerols in natural lipidic matrixes. J Chromatogr A. 2006;1112:269–275.
17. Mondello L, Tranchida PQ, Stanek V, Jandera P, Dugo G, Dugo P. Siver-ion-reversed-phase comprehensive two-dimensional liquid chromatography combined with mass spectrometry detection in lipidic food analysis. J Chromatogr A. 2005;1086:91–98.
18. Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306.
19. Kalili KM, de Villiers A. Recent developments in the LC separation of phenolic compounds. J Sep Sci. 2011;34:854–876.
20. Maiani G, Castón MJ, Catasta G, et al. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res. 2009;53:S194–S218.
21. Dugo P, Herrero M, Giuffrida D, Ragonese C, Dugo G, Mondello L. Analysis of native carotenoid composition in orange juice using C30 columns in tandem. J Sep Sci. 2008;31:2151–2160.
22. Dugo P, Herrero M, Giuffrida D, Kumm T, Dugo G, Mondello L. Application of comprehensive two-dimensional liquid chromatography to elucidate the native carotenoid composition in red orange essential oil. J Agri Food Chem. 2008;56:3478–3485.
23. Li XQ, Zhang F, Sun YY, et al. Accurate screening for synthetic preservatives in beverage using HPLC-TOF-MS. Anal Chim Acta. 2008;608:165–177.
24. Yang DJ, hen B. Simultaneous determination of nonnutritive sweeteners in foods by LC/ESI-MS. J Agri Food Chem. 2009;57:3022–3027.
25. Zygler A, Wasik A, Namiesnik J. Analytical methodologies for determination of artificial sweeteners in foodstuffs. TrAC Trends Anal Chem. 2009;28:1082–1102.
26. Herrero M, Simó C, García-Cañas V, Ibáñez E, Cifuentes A. Foodomics: MS-based strategies in modern food science and nutrition. Mass Spec Rev. 2012;31:49–69.