CHAPTER 4
Viral Trojan Horse
AZT is a hot button for many people living with HIV. It’s an ugly part of our history, a drug that revealed unfairness in our drug development industry, homophobia in our government, and a lack of empathy in those who should have been protecting the public. HIV researchers tend to have a different view of the drug. For them, it represents the first hope, the forefather of all HIV drugs to come. Today, it’s one of the few drugs considered safe for an unborn child.
In 1984, Burroughs Wellcome Company, the company that came to make windfall profits from AZT, was the twentieth-largest pharmaceutical company in the United States. David W. Barry, vice president of research, specialized in viral diseases. It was a time when few companies researched viruses, which are notoriously difficult to target. Unlike bacteria, viruses form an intimate connection with an invaded cell’s machinery. Similar to cancer, it is almost impossible to kill a virus without killing the cell as well. Barry was particularly interested in AIDS, the new disease that had been discovered only three years before.
Although it’s hard to imagine a pharmaceutical company taking such risk, in 1982, Barry formed a small group to begin considering what drugs could treat the new disease, then briefly known as gay-related immune deficiency, or GRID, which had no known cause. It was a bold move. No other pharmaceutical company would remotely consider devoting resources to this disease. Barry found himself drawn to working on the disease despite, or perhaps because of, the enormous challenges it presented. Those closest to Barry found his interest in AIDS bordering on obsession. Early in 1984, French and American scientists had, at almost the same moment, established that AIDS patients were infected with a retrovirus.
HIV is the retrovirus they identified. It is a kind of viral Trojan horse. It is “retro” because it reproduces itself in a way that’s backward from how most life-forms manage this wonderful trick. In the nucleus of each cell in our body, and we have an awful lot of them, the genetic recipe for building our entire body can be found. Every cell has the complete list of instructions. Those genes are packed in DNA, tightly coiled molecules of nucleic acids. To make use of all the instructions in our genes, certain enzymes unwind the DNA helix in the cell’s nucleus. But to ensure that the precious DNA is not lost, another enzyme swoops in and makes a copy of the DNA needed. This is called transcription. This copy is made literally backward and has a few special alterations. (One of these alterations is to a base called uracil, a molecule whose origin may be extraterrestrial—yes, really.) The copy is called ribonucleic acid, or RNA. The RNA blueprint is then transported from the nucleus to a ribosome, which, once it gets its hands on the RNA, translates the backward code and makes proteins from the blueprint.
DNA 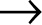 RNA
RNA 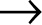 Protein
Protein
Simple as one, two, three. For decades, this definition of the process, made by Francis Crick, the codiscoverer of DNA with James Watson, was considered scientific dogma. That is how life works. So imagine how surprising it was when scientists discovered that a virus could run in the opposite direction. Retroviruses forced scientists to question what defines life. Here was an organism with a genetic code of its own but with no cell to house it. Can you be alive if you hold the blueprint to life but have to borrow the construction plant from another organism? Can there be life outside a cell? Taxonomists have spent decades debating this, perhaps pointlessly. As Carl Zimmer argues in A Planet of Viruses, “Trying to find a moment in time when such RNA-life abruptly became ‘alive’ just distracts us from the gradual transition to life as we know it.”
This is what retroviruses do. They trick our cells into making the proteins they need, as directed from their RNA. Instead of starting with DNA, HIV holds all its genetic information as RNA. The virus exists as two simple strands of RNA packaged into a spiky protein shell containing all the enzymes it needs. The viral RNA travels into the nucleus of our cells and instead of performing transcription, it performs reverse transcription: HIV uses an enzyme called reverse transcriptase to transform a copy of its RNA into DNA (the same format as the DNA in our own human cells). By converting its RNA to DNA, it can insert its genetic material into our own. Because all the virus’s genetic material is now DNA, our immune system can’t distinguish between viral genes and our own human genes. Once it has done this, the virus has essentially tricked our cells into making the proteins that HIV needs to make more virus.
RNA 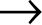 DNA
DNA 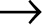 RNA
RNA  Protein
Protein
After the virus has used reverse transcriptase to make a DNA copy of its own RNA, it still needs to hide it in our DNA. To do this, it uses another enzyme, integrase, to assimilate the newly made DNA into human genetic material. Integrase cuts into our DNA and then joins the cut strands of our chromosome with the newly made viral DNA. This step is irreversible; once the process is complete, the virus will always have a place in our chromosomes.
As directed by the virus, the invaded cell produces long, unwieldy chains that incorporate the viral enzymes: reverse transcriptase, integrase, and protease. Like a salad, these proteins have to be chopped up and mixed together to make a virus. The virus uses the enzyme protease to do this. Without protease, the virus would have all its basic components but would not be infectious. After the protease finishes dicing proteins, the virus undergoes its final assembly, bringing together single-stranded RNA, viral enzymes, and core proteins to form a capsid, that is, a protein shell containing everything it needs except the viral envelope. The virus picks up this last piece of the viral puzzle as it is leaving the human cell. The envelope—the proteins that surround the virus—is part virus and part human. Because the virus has this unique coating, it can go on and infect another cell. This life cycle is shown in the illustration on page 37. A mature virus particle, or virion, is born.
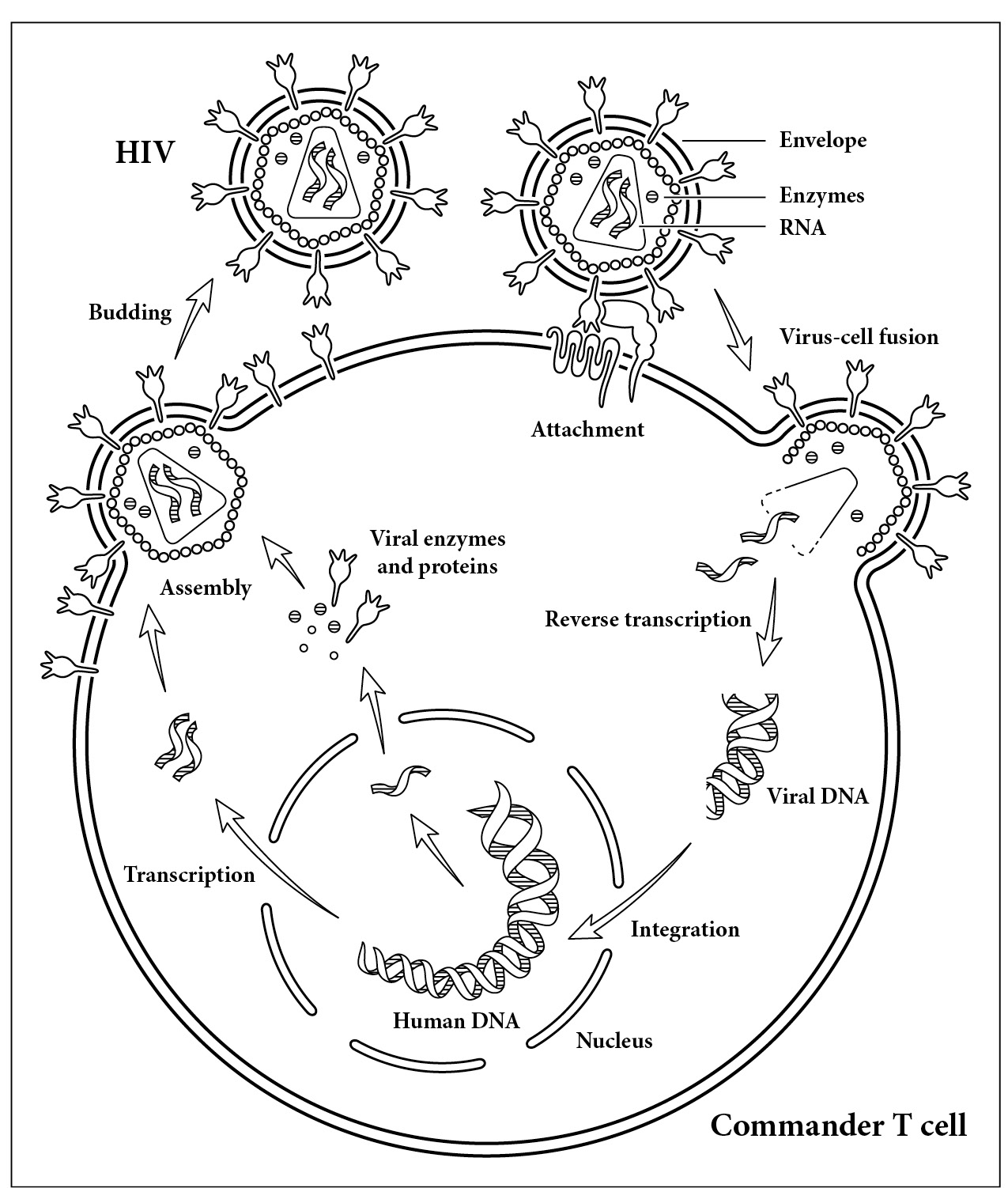
How HIV invades a cell. The virus first makes contact with a T cell, releasing its enzymes and RNA inside. The reverse transcription enzyme translates the viral RNA into DNA. The virus then makes its way to the nucleus, where the integrase enzyme hides the viral DNA within our human DNA. The viral DNA is transcribed back into RNA by the cell. Our cell then makes viral proteins as directed by HIV. The protease enzyme assembles these proteins into a virus. As the virus leaves the cell it picks up proteins from our cell membrane, giving it the key to unlock more T cells.
It’s hard to wrap our minds around how small HIV is. At four-millionths of an inch, the tiny intruder is one-twentieth the size of a bacterium, one-seventeenth the size of the T cell it invades. The virus is a thousand times thinner than a human hair. Yet it has a big footprint, capable of making billions of itself daily. This sea of invaders completely overloads the human immune system, ultimately resulting in widespread death of the very cells the virus needs to sustain itself. Killing our cells is ultimately not wise for the virus. Unfortunately, by coincidentally killing the cells we need to protect ourselves, it kills us, too.
Retroviruses have lived within our bodies for millions of years. They’ve left behind an archaeological record of sorts, traces of viral DNA hidden within our own genome, impossible to wipe clean. As the ancient viruses invaded our chromosomes, they left behind pieces of themselves, a historical record of infectious disease. More than a historical record, the viruses are a part of our genetic code, influencing our progress as a species. This influence can be wielded by retroviruses. Other viruses, such as Spanish flu and yellow fever, may take the lives of millions; they may even change the course of history, but only a select group of viruses can conquer who we are and what makes us human.
We think of retroviruses as faceless monsters, destroyers of life. But not all retroviruses harm their host. What is the difference between the retroviruses that destroy and the ones that do no harm? The answer seems to be evolution. Two retroviruses that can live peacefully with their animal hosts are closely related to HIV: simian immunodeficiency virus (SIV) and feline immunodeficiency virus (FIV). In FIV infection of cougars, where the virus has a long shared history, the infection causes no disease. However, once transferred to domestic cats, where the virus has a much shorter evolutionary history, FIV can cause AIDS-like symptoms. A similar scenario exists in monkeys. Some species, such as African green monkeys, live in relative harmony with their SIV, harboring the virus with little consequence. These monkeys have likely lived with their SIV for millions of years, plenty of time for both the animal and the virus to find the right balance. Contrast this with the consequence of an SIV that was transferred to humans: HIV. HIV has had only a short time to get to know us, approximately one hundred years. We’ve known HIV even a shorter amount of time, about thirty years. If we could wait a million years, perhaps we could achieve a truce with HIV. Since viruses are ultimately driven by biology to make more of themselves, the best way to make more of themselves is to keep us, like the African green monkeys, alive and replicating. Ironically, the only way HIV can succeed as a virus is to allow our survival.
HIV is not one uniform virus. It swarms in our bodies but varies genetically from particle to particle. When the virus makes DNA from its RNA, the product is riddled with mistakes, and this gives it a distinct advantage in being able to adapt and mutate. The virus’s poor ability to precisely copy itself makes it, as a swarm in the body, more resilient. That’s why there is such a high level of drug resistance to HIV. Even though a drug may be able to efficiently attack one part of the virus, somewhere in that swarm probably exists a lone wolf able to evade the drug. That variant will begin to replicate until it can overcome the drug’s affect. It is this unique property of HIV that makes it so difficult for us to develop effective drugs to fight it and the reason new antiviral drugs are constantly in development.
Almost every person infected with HIV, if they don’t receive treatment, will progress to AIDS. HIV wears down the immune system, killing off our immune cells, particularly our CD4 T cells. Without these T cells, the commanders of the immune system, we are vulnerable to diseases we normally would be able to defeat. Everyone advances at a different pace; for some it may be decades, and for others only weeks. On average, for a person off therapy, it takes 10 years to go from HIV infection to AIDS. For this reason, AIDS is defined either by the loss of the commander cells—less than 200 of them in a microliter of blood (we normally carry between 500 and 1,000 per microliter)—or by the presence of an AIDS-defining illness. Defining illnesses, rarely seen in healthy people but common in persons with AIDS, include a bacterial pneumonia and a herpes-driven tumor that causes lesions across the body. The presence of one of these diseases indicates that the immune system has broken down and left the body defenseless. Beyond its clinical definition, AIDS carves a wide path in the body. Extreme fatigue and a wasting syndrome characterize the disease. Those with AIDS resemble cancer patients, with sunken cheeks and slim bodies. For those who evade death, the disease still carries unshakable stigma.
• • •
The discovery of the reverse transcriptase enzyme in 1970 flew in the face of everything scientists thought they knew about DNA. The discovery came from two independent research groups: geneticist Howard Temin with his postdoctoral fellow Satoshi Mizutani at the University of Wisconsin–Madison, and biologist David Baltimore, a young investigator at the Massachusetts Institute of Technology. Fifteen years before the discovery of HIV, Baltimore was studying a lesser-known retrovirus, rous sarcoma virus, when he came across the unique enzyme. It was a watershed moment in virology. These men would share the Nobel Prize for their groundbreaking work only five years later. For Baltimore, it was just the beginning of his lifelong investigation of retroviruses. The discovery of reverse transcriptase was a pivotal moment for molecular biologists and, although they couldn’t know it at the time, for HIV therapy. Because of this discovery, inhibitors for an enzyme essential to HIV were already in development thirteen years later when the new virus, HIV (initially named human t-lymphotropic virus type III, or HTLV-III), was found. But the road to effective pharmaceuticals would be a tortuous one.