CHAPTER 9
But, Doctor, I Don’t Feel Sick
In 1996, just as today, experts had not agreed upon the best time for a patient to start treatment with antiviral drugs. Although some scientists hypothesized that early therapy could confer benefits, there was no hard evidence. Many patients had difficulty tolerating the drugs, which caused a range of side effects from psychiatric disturbances to gastrointestinal distress to fat redistribution. At St. Clare’s Hospital in Hell’s Kitchen in New York City, the AIDS ward was packed with young men and women suffering from all these side effects. One young man craved only ice, unable to tolerate solid food. Another lived in a state of dementia, confused and hallucinating. Almost everyone exhibited the sunken cheeks that marked a person, as prominent as a scarlet letter, as HIV-positive. Because of this range of side effects, physicians could not depend on universal guidelines, and instead had to judge for themselves whether to start therapy right away or wait until the effects of the virus could be measured in the patient.
HIV infects the majority of people as a single virus. It enters a cell and begins to invade. Its victims are T cells, a type of white blood cell. No matter how the infection occurred, the invasion begins primarily in the intestines and rectum. We tend to think of HIV as a disease in the blood, since the majority of research and testing has focused on this part of the body. Actually, most viral replication takes place in the intestines and rectum, where a dense network of white blood cells, including T cells, reside. The gut contains the vast majority of the body’s immune system, more than 70 percent of all T cells reside there, not in our blood. The gut is the battleground for HIV and a broad range of infections. Following ingestion, sexual transmission, and even intravenous transmission, the gut is the first place HIV takes on the immune system. It isn’t clear why this is so in the case of non-anal sex and intravenous infection, but the explanation may lie in our immunological past.
HIV is able to break into T cells because of the proteins the cell holds on its surface. HIV needs two proteins to sneak into the cell. The first is CD4. T cells with the protein CD4 are the commanders of the immune system. They coordinate the attack and send in the killer T cells, the storm troopers of the immune system, to clear the virus. The fact that HIV first identifies and takes out the commanders is a shrewd tactical strategy, for without its commanders, the immune system can no longer coordinate its attack on HIV.
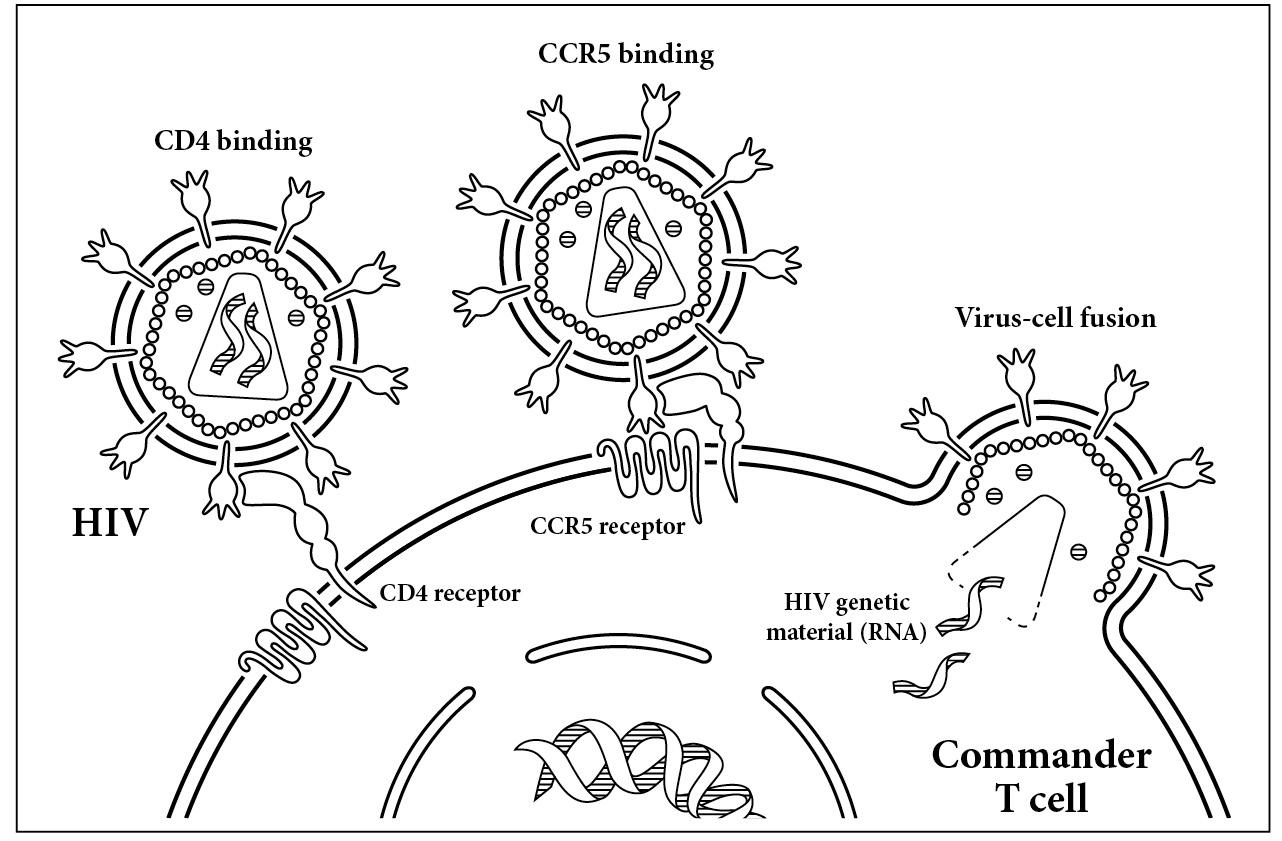
The key to unlocking a T cell. HIV’s envelope protein first makes contact with the CD4 receptor on the surface of the T cell. Once it has bound to this receptor, it then binds a co-receptor, CCR5. This interaction causes the envelope protein to fold in on itself, bringing the virus close to the cell. Once virus and human touch, the membranes fuse, allowing the virus to enter the cell.
But HIV needs more than just CD4 to enter T cells. The presence of a second protein, CCR5, is critical. The vast majority of viruses need CCR5 to enter our cells. This human protein serves no real purpose in our bodies. Like our appendix, its presence or absence doesn’t seem to affect our health. The CCR5 protein lies next to CD4 on the cell surface. Like opening a locked door, HIV’s contact with CD4 and CCR5 acts as a key fitting a lock. As shown in the illustration below, the virus first forms a tight bond with the CD4 protein on the surface of the cell. Then it also grasps CCR5.
The part of HIV that is the key to this unusual lock is an ingenious tool. Each particle of HIV is covered in little spikes, which are the envelope protein of the virus. Each of the spikes is needed to get into the T cell. The spikes themselves are split into two distinct units: gp120 and gp41. The gp120 unit is located on the tip of the spike, while gp41 is at the bottom. As the virus approaches the cell, either free-floating in our blood or trapped inside the tissue of our gut, the top part of the spike, gp120, binds to CD4. When that initial contact is made, the virus is drawn close to its cell victim. The bottom of the spike is held in just the right spot as it makes contact with CCR5, the close neighbor of CD4.
Once HIV holds both CD4 and CCR5, the bottom part of its envelope spike begins to fold in on itself. This folding draws the virus close to the cell so that the membranes of the two are flush against each other. Like two drops of water running down a window, the virus and the cell finally meet, and their membranes mesh. The two drops become one. The virus is now able to spew its contents into the human cell. Those viral contents are the RNA and all the enzymes needed to unpack themselves and move into the cell. Once inside, the viral RNA travels right to the nucleus of the cell, ready to take over our DNA and cell machinery and begin copying itself.
There are only a few types of cells that carry both CD4 and CCR5. While HIV can infect any of them, we tend to focus on the loss of the commanders since they are both abundant in the blood and critical to our ability to defend ourselves. Historically, we tend to think of the disease as one of the blood despite the fact that the virus lives in our tissues as well. There’s a reason for this. It’s easier for us to draw blood and measure the commander cells than it is to excise tissue and measure the other cell populations that carry CD4 and CCR5.
The viral envelope scans the cells that surround it, detecting the CD4 and CCR5 proteins that poke their heads out of our cells. Once it detects these proteins, it binds to them like a magnet, gaining entry into the cell. Apart from commanders, HIV attacks cells called macrophages, white blood cells that ingest invading pathogens. Macrophages are sometimes called the garbage disposal unit of the body. HIV doesn’t kill macrophages directly. Instead, it keeps the cells alive, even going so far as to change the way our body communicates with these little garbage disposal units. The strategy is ingenious, for macrophages can travel anywhere in the body, carrying the virus with them. HIV is most famous, though, for its destruction of T cells, the cells that regulate our immune system.
Commander T cells are perfectly round and covered in fuzzy spirals of the CD4 protein. Again, these commanders of the immune system don’t directly kill cells infected by virus or bacteria. Instead, they coordinate the response to the infection, activating the storm troopers, or killer T cells, that, as the name implies, directly kill cells infected by the virus. The commanders also activate the B cells, which, like a bomber squad, drop antibodies on the virus, mangling it and making it difficult to infect new cells. In each cubic millimeter of blood, about the size of a raindrop, the average person maintains a healthy level of 500 to 1,500 commanders, but at the height of HIV destruction, this number can drop to zero. On the road to zero, HIV kills a lot of T cells. HIV acts like a trained assassin, killing the commanders that the rest of the military depends on.
But here’s the rub. During acute infection, when the newly invading army, HIV, is rallying its troops, there are actually few symptoms, and they are all mild. Patients experience flu-like symptoms common to viral infections, such as fever, achiness, and fatigue. This stage is not about killing T cells, although many will perish, mostly in the tissues. These losses are minor compared to the massacre about to occur in the weeks ahead. The commander cells aren’t noticing anything unusual going on. During this time, the virus is ramping up, making as much of itself as it can, on average 10 billion copies a day. Two critical events are linked: a peak of virus occurs in the body just before the number of commanders plummets. Externally, the person looks healthy, and likely feels healthy, but inside, their immune system is crumbling.
Within the first few weeks of infection, the virus kills cells locked away in our tissues. These cells, comprising both commander T cells and macrophages are, unlike in the blood, packed closely together. They are the perfect first victims for the virus. We don’t typically measure the cells that line our gut and genital tissue. From a medical perspective, we’re not likely to notice that they’re gone. Once the virus kills these cells, the cells don’t come back. Even after decades of antiviral therapy, we can’t replace this precious hoard of commanders. As the virus makes more and more of itself, it seeps into our blood. There is no magic switch we know of that occurs to mark the end of acute infection and the beginning of chronic infection, which can lead to AIDS. Instead, the virus seems to reach a critical mass, then the blood is filled with billions of copies of the virus. The immune system responds but is overwhelmed. The destruction begins. While the commanders are the first victims, their decimation takes time. As cells are killed, the immune system is left defenseless. And just like that, a person progresses from HIV to AIDS.
So when should a doctor begin therapy? Early on, to prevent damage to the immune system caused by the virus? Or later, after the effects of the virus can be measured in the blood and the need for medication is obvious? The drugs prescribed remain the same whenever therapy is started. However, the general wisdom around 1996 was that, once started, HIV medications should never be stopped. Stopping the drugs could open up the virus to mutation. And once mutated, the virus could develop resistance to the antiviral drugs.
Doctors hate to start patients on therapy when there is no sign of disease. With no evidence that early therapy held any benefit, there was simply no reason to start therapy before symptoms developed. In fact, it was risky to start therapy too soon because patients might be tempted to stop the medication and then develop drug resistance. After all, it’s asking a lot of someone to take medication when they don’t even feel sick—particularly when that medication isn’t easy to take and causes awful side effects. So while in 1996 we finally had effective, new drugs for HIV, what we didn’t have was the instruction manual. We were about to get a sense of just how much the burgeoning science of personal genetics could help us in the search for a cure for HIV.