Note: Page numbers in italics indicate illustrations.
Aaron Diamond AIDS Research Center, 68, 95–96
ablative therapy, 68, 254, 261
academic publishing, 172–74, 178–79, 202–3
acquired immune deficiency syndrome (AIDS), 40, 68, 87, 92–93, 233
acquired immune system, 24
The Act of Creation (Koestler), 183
ACT UP, 11
activism and advocacy, 11, 53, 56, 57–63
Acute HIV Trials Group (ACTG), 190–91, 197
acute lymphoblastic leukemia (ALL), 221
acute myeloid leukemia (AML), 120–21, 155–56, 200–201, 203, 251, 261
acute stage of HIV infection
and drug trials, 190, 193, 195, 197
and early treatment approach, 68–69, 187–88, 193, 195, 256
and the infection process, 5, 91–92
symptoms of, 14–15
adenine, 47–48
aging and HIV, 214–15
AIDS Coalition to Unleash Power, 61
AIDS Research Center, 76
American Cancer Society (ACS), 46
American Society for the Control of Cancer, 45–46
And the Band Played On (Shilts), 31
Ando, Dale, 227, 229
Andrew (HIV patient)
and drug trials, 83–84, 172
and experimental treatments, 18, 111, 114–15, 131–32, 150
HIV diagnosis, 3–4, 6, 10–12, 14
Jessen’s relationship with, 207–8, 270
animal research, xiii–xvii, 48–49, 127, 231–35, 237–38, 250
antibodies, 15, 171, 176
antigen-presenting cells (APCs), 80, 81, 107–9, 108
antigens, 24, 81, 82, 107, 108
antisense oligonucleotides, 150
antiviral and antiretroviral drugs
and advances in HIV treatments, 95
and bone marrow transplants, 157
costs associated with, 212–13
difficulties tolerating, 214, 262
and drug trials, 194
and elite controllers, 77
and fiscal motivations, 240
and latent virus, 5
and research accidents, xvi, xvii–xviii
antiviral and antiretroviral drugs
resistance to, 39–40, 58–59, 93, 196
and success of Hahn’s treatment, 165–66
See also early treatment approach; side effects of drugs
artificial dendritic cells, 226–27
Atripla, 213
autoimmune disorders, 109
AZT (azidothymidine)
and AIDS activism, 61
and antiviral drug research, 33, 49, 53, 55–56, 59–63
and cancer research, 112
and combination therapies, 84
cultural significance of, 57–58
drug trials, 58–60
and d4T, 192–93
and early HIV cases, 11
and HIV in infants, 241
and politics of HIV research, 57–63
shortcomings of, 83, 131–32
side effects of, 98
B cells, 24, 74, 91, 223
B*27 gene, 107
B*57 gene, 107
bacterial infections, xx, 40, 55, 228
Baltimore, David, 41, 257, 258
Barry, David W., 33–34, 49, 54
Berg, Jeremy, 227
Berlin, Germany, 9–10, 16–17, 21–22, 84
Berlin patients. See Brown, Timothy Ray; Hahn, Christian
biopsies, 161, 176, 261, 263–65
Birth Control Federation of America, 45
Black Death (bubonic plague), 67, 130
blasts, 156
blood-brain barrier, 214–15
Blue, Eric, 243
Bolognesi, Dani, 55
bone marrow
and acute lymphoblastic leukemia, 221–22
and AZT’s side effects, 59
and Brown’s treatment and cure, 153–62, 176, 253–55, 261
and cancer treatments, 120–21
donors and transplants, 123–25, 128–29, 153–62, 212, 239
early research on, 224–25
and the immune system, 24
and latent virus, 5
risks associated with transplants, 212
boy-in-the-bubble disease, 62, 235
brain trauma and infection, 160–61, 175, 214–15, 261
Breaking Bad News, 15
Breton, Louis, 258–59
Bristol-Myers Squibb, 190–93
Broder, Sam, 49–50, 53–55, 60, 62–63
Brown, Timothy Ray
background, 21–22
biopsies on, 255
and bone marrow transplants, 153–62, 253–55, 261
and cancer diagnosis, 119–23, 221–22
and the Conference on Retroviruses and Opportunistic Infections, 179–81
as conference subject, 241–42
connection with Hahn, 20
contrasted with Hahn’s case, 267–70
and experimental treatments, 131, 133, 144–46, 175–77
and family support, 141–42
and functional cure, 202–3
and gene therapy strategies, 224–25, 236, 238
and HIV diagnosis, 22–23, 26–29, 98, 144
and HIV treatment methods, 125–30
impact on HIV research, 209, 211–13, 240–44, 251–55, 256–57, 259, 261–62, 261–67
and patient anonymity, 203
and treatment interruption strategy, 195
variety of treatments used on, 217–18
bubonic plague (Black Death), 67, 130
budding, 37
Burroughs Wellcome Company, 33, 49, 54, 60–62
Bush, George, 251
B.Z. (tabloid), 185, 206
cachexia, 144
Café Einstein, 22, 28
California HIV/AIDS Research Program, 250
California Institute for Regenerative Medicine (CIRM), 251, 259
Calimmune, 258–59
cancer and cancer research
and bone marrow transplants, 153–54, 155–60
and Brown’s case, 119–23, 221–22
and cachexia, 144
cancer screens, 103–4
and the delta 32 mutation, 98
and drug development, 112–13
and early treatment approach, 69
and elite controllers, 102
and gene therapy strategies, 200, 231
and histone deacetylase, 246
impact on HIV research, 43–56
and killer T cell response, 79–80
See also leukemia; specific types of cancer
Cannon, Paula, xvii, 249, 251, 259
capsid, 36
CART-19, 222
CCR5 protein and receptor
and artificial dendritic cells, 226–27
and CXCR4-using virus, 176–77
and the delta 32 mutation, 95–96
and gene therapy strategies, 179–80, 200, 201, 229–31, 230, 235–36, 238
and gut tissues, 105
and HIV infection process, 88–89, 89, 90, 125–27
and ongoing HIV research, 270
and small interfering RNAs, 258
and stem cell transplants, 203, 253
and therapy options, 127–30
and zinc finger nucleases, 249–50
See also delta 32 (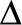 -32) mutation
-32) mutation
CD4 protein and receptor, 40, 69, 88–91, 89, 99–100, 105, 125, 176–77, 233, 235
cell division, 47, 112–13
cellular mitochondria, 144–45
Chandrasegaran, Srinivasan, 227
Charité Hospital
and Brown’s cancer diagnosis, 119
and Brown’s HIV diagnosis, 23, 27
closure of transplant program, 269–70
and Hütter’s career, 122
and Jessen’s health, 207
and stem cell transplants, 124–26, 130, 158, 160–61
chemokines, 96, 263
chemotactic cytokines, 96
chemotherapy
and “ablative therapy,” 68
and bone marrow transplants, 59–60, 155, 157, 221–22
and Brown’s treatment and cure, 119–20, 122–23, 128, 155, 157, 159, 253–54
and the CCR5 gene, 125
and cord blood, 244
and Hahn’s treatment and cure, 137
physical toll of, 261
Chen, Irving, 258
Children’s Hospital of Philadelphia, 222
chimeric antigen receptor (CAR), 223
cholera, 67
chromosomes, 36
chronic HIV infection, 92, 187, 190
combination therapy, 84–85
commander T cells
and CCR5 cutting ZFNs, 235
and the ELISPOT assay, 172
and elite controllers, 99–101
and HIV infection process, 37, 89, 91
and HIV-specific responses, 187
and the immune response, 82, 108
and success of Hahn’s treatment, 163–64
Concorde drug trial, 83
conditioning regimes, 159, 201, 217, 253
Conference on Retroviruses and Opportunistic Infections (CROI), 177–80, 199–203, 211, 241, 247–48
cord blood, 243–44
Cotton, Deborah, 193
counseling, 15, 27
crippled virus, 168
CXCR4 receptors, 126–27, 158, 177, 180–81, 263, 265
cystic fibrosis, 102
cytidine, 47–48
cytokine, 171
Deeks, Steve, 199, 263, 264, 266
delta 32 (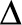 -32) mutation
-32) mutation
and bone marrow transplants, 157, 158
and Brown’s treatment and cure, 217, 238, 253
and the CCR5 receptor, 176–77
and cord blood, 243–44
and CXCR4 viruses, 158, 180, 263
described, 95–98
evolution of, 130
and HIV resistance, 103, 124
and ongoing HIV research, 270
and stem cell transplants, 125, 128, 157, 180, 203
dendritic cells, 226–27
deoxyribonucleic acid (DNA)
and cancer research, 47
and histone deacetylase inhibitors, 245–46
and HIV infection process, 34–40, 37
and latent virus, 246
and retroviruses, 34
and zinc finger nucleases, 228–29
deoxyribonucleotides, 112
Der Spiegel, 16
d4T, 192–94, 197
diagnosis of HIV
Andrew’s diagnosis, 10–12
Brown’s diagnosis, 22–23, 26–29, 98, 144
and cancer research, 43
and early treatment approach, 18–20, 188–89
enzyme-linked immunosorbent spot assay (ELISPOT), 170–72
and funding for HIV research, 52–53
and the gay community, 144–46
and gene therapy strategies, 201
and Jessen’s practice, 13–17
ongoing testing, 151–52
physical symptoms, 159–60, 213–15
reactions to positive results, 27–29
and research on cured patients, 263–64
sharing diagnoses, 142–44
and success of Hahn’s treatment, 165–68
test options, 6–7, 24–27
didanosine (DDI), 18, 135, 189–90, 192, 194
drug resistance, 39, 58–59, 93, 196
drug treatment schedules, 135–40, 148–49, 190
early treatment approach
acute stage of HIV infection, 68–70, 187–88, 193, 195, 255–56
conflicting opinions on, 87, 136–37, 245
and drug trials, 249
and elite controllers, 171
and HIV in infants, 241–42
and hydroxyurea, 132
and large-scale clinical trials, 216–17
and long-term consequences of HIV infection, 215–16
and success of Hahn’s treatment, 169–70
and treatment interruption strategy, 195
ELISA test, 24–26
ELISPOT test, 170–72
elite controllers
contributions to HIV research, 105–6
and crippled virus, 168
and early treatment approach, 171
and human leukocyte antigen, 101–2, 106–9, 108, 217–18
and ongoing HIV research, 256, 266–67, 269
and treatment interruption strategy, 187, 194
viral loads of, 104–5
and Walker’s research, 76–77
embryonic stems cells, 122
“The End of AIDS?” (Newsweek article), 70
enzyme-linked immunosorbent spot assay (ELISPOT), 170–72
epididymitis, 136, 137
ethical issues in HIV research, 114–15, 188
evolution, xx, 38–39, 129–30, 232
exposed uninfected (EUs), 96. See also elite controllers
family doctors, 113, 216
fat redistribution (lipodystrophy), 144–45
Fauci, Anthony
and early therapy strategy, 242
and media coverage of HIV research, 211
and stigma associated with HIV, 50
and treatment interruption strategy, 196
and Walker’s research, 75–76
feline immunodeficiency virus (FIV), 38–39
“Fire with Fire” (video), 223–24
fiscal motivations in HIV research, 193, 198, 212
Forich, Dan, 103, 105–6, 109
Foundation for AIDS Research (amFAR), 199, 259
Fox, Cecil, 167–69
functional cures, xix, 194, 202, 215, 239, 241, 245, 251, 267
funding for HIV research, 173, 239, 259, 269
gag gene, 170–71
Gallo, Robert, 50–52, 62, 72, 111–13, 149–50, 209
Gates, Bill and Melinda, 269
gay-related immune deficiency (GRID), 34, 50, 71
gene therapy strategies
author’s experience with, xiv
and clinical trials, 235–40
and elite controllers, 102–3
and fiscal motivations, 259
HIV used in, 222–23
and human trials, 250–51
and humanized mouse model, 250
and intellectual property issues, 227
and Lisziewicz’s research, 149–52
mechanics of, 200–201
and media coverage of research, 202–3
and ongoing HIV research, 255
and reverse transcriptase, 258
and treatment interruption strategy, 237
and zinc finger nucleases, 228–31, 230
genetics
and the delta 32 mutation, 95–98
and elite controllers, 76–77, 99–102, 106, 217
gene sequencing, 102, 176, 265
and HIV infection process, 34
and human leukocyte antigen (HLA), 75
and killer T cells, 76
personal genetics research, 93
See also delta 32 (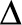 -32) mutation; gene therapy strategies
-32) mutation; gene therapy strategies
GlaxoSmithKline, 62
global pandemics, 65–77
Goodbye to Berlin, 1
gp41, 89
gp120, 89–90
graft-versus-host disease, 128, 212, 217, 243–44, 253
grants for HIV research, 259
Gregory, Philip, 229
guanosine, 47–48
gut tissues, 5, 88, 104–5, 176–77
Hahn, Christian
academic attention to case, 209–10
case as model for clinical trials, 215–18
Hahn, Christian
contrasted with Brown’s case, 267–69
decision to end drug therapy, 151, 153–54
and the delta 32 mutation, 98
and drug treatments, 131–34, 135–40, 148–49, 192–94, 197
and early treatment approach, 190
and family support, 142–43, 147
and HIV diagnosis, 13–20, 24
as inspiration to researchers, 242
and media coverage of HIV research, 202, 209, 245
and ongoing HIV research, 248, 256–57, 263–64
and success of treatments, 163–74
and tabloid articles, 185
and treatment interruption strategy, 77
Heckler, Margaret, 51
helper T cells, 80
hematopoietic stem cells, 123–24, 201, 243, 249–50
hemoglobin, 59
Henrich, Timothy, 252–53
hepatitis, 79, 137–38
herpes, 40, 66–67
heterozygous mutations, 97, 238
highly active antiretroviral therapy (HAART), 84–85
histone deacetylase inhibitors, 245–47, 255, 257
“HIV Eradication by Stem Cell Transplantation: Is It Feasible?” (Hütter), 211
HIV-specific T cells, 194–95
HLA typing, 74–75
HLA-B genes, 107
Ho, David
background, 65–66
and the delta 32 mutation, 95–96, 98
and drug trials, 82, 84–85
and early HIV research, 72
and early treatment approach, 16, 132, 188–89
and treatment interruption strategy, 77
and vaccine research, 68
Hofmann, Wolf, 178
homophobia, 33, 52
homozygous mutations, 97
Horwitz, Jerome, 46–49, 55–56, 62, 70, 125, 192, 241
HTLV-III, 50
human leukocyte antigen (HLA)
and elite controllers, 101, 106–7
HLA typing, 74–75
HLA-B genes, 107
and the immune response, 80–82, 81, 108
and immunology research, 74–75
subtypes of, 217–18
humanized mouse model, 231–35, 237–38, 250
Hütter, Gero
background in HIV research, 249
and bone marrow transplants, 156–58, 160
and Brown’s cancer diagnosis, 119
and Brown’s cure, 218
and cancer treatments, 121–22
and challenges of detecting HIV, 265–66
and conference presentations, 176–80, 199, 211
and critiques of research, 209–11, 212
and critiques of treatment methods, 263
and the delta 32 mutation, 97–98
and gene therapy strategies, 201–3
and HIV treatment methods, 124–29
influence on HIV research, 253–54, 261, 269–70
professional style, 121–22, 124
hydroxyurea
background of, 112–13
and the “compassionate use” exemption, 131–34
conflicting opinions on, 186–87
and drug schedules, 18
and drug trials, 189–94, 197–98
and early treatment approach, 137
and gene therapy strategies, 151, 173
and ongoing HIV research, 271
and treatment interruption strategy, 197
immune system
and bone marrow, 121
described, 79–82
and elite controllers, 107–9, 108
and HIV infection process, 38, 40, 87–93, 89, 99
and HIV tests, 25–26
and human leukocyte antigen (HLA), 74–75, 107–9, 108
innate immune system, 24
and success of Hahn’s treatment, 169
and treatment interruption strategy, 195–96
indinavir, 18, 19, 190
infection process, 38, 40, 87–93, 89, 99, 125–27
inflammatory disease, 234
influenza, 4
informed consent, 106, 114
Institute of transfusion Medicine and Immunology, 270
Institute of Tropical Medicine, 23, 27
integrase, 36
intellectual property issues, 227–28
interferon-γ, 170–72
International AIDS Conference, 75, 79, 256
Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), 191
intestines, 88. See also gut tissues
irradiation, 159, 161
Isherwood, Christopher, 1, 21–22
Jason (HIV patient), 213–14
Jenner, Edward, 67, 68
Jessen, Arne, 8, 83–84, 207
Jessen, Heiko
and drug treatments, 83–84, 113–15, 131–34, 148–52, 189–93
early experience with HIV, 3–4, 6–12
and early treatment approach, 136
and elite controllers, 106
family support, 143
and gene therapy strategies, 158
and HIV diagnoses, 13–20, 24, 27
and hydroxyurea trials, 189, 197–98
and the International AIDS Conference, 79
legal and personal troubles, 206–8
and media coverage of HIV research, 185–86, 201–2, 205–6
and ongoing HIV research, 245, 249, 268, 270–71
professional style, 121–22
and publishing conflicts, 178–79
relationship with Andrew, 111
relationship with Gallo, 111–12
relationship with Jessen, 216
and research authorship issues, 173–74
and success of Hahn’s treatment, 163, 165–69, 171–74, 218
June, Carl, 222–24, 226–27, 231, 235, 239–40, 251
Kaposi’s sarcoma (KS), 66–67, 144, 145
Kauffman, Ross, 223–24
killer T cells. See storm trooper (killer) T cells
Koestler, Arthur, 183
Kuritzkes, Dan, 253
lamivudune, 241
The Lancet, 247
Lanphier, Edward, 227, 228
Lasker, Albert, 45
Lasker, Mary, 44, 49
latent HIV infection, 5–6, 200, 246–48, 254
Laurence, Jeffrey, 199
Lee, Harper, 117
leukemia, 48, 68, 120–22, 153–61. See also acute myeloid leukemia (AML)
Levine, Bruce, 225–27
Lewin, Sharon, 248
life expectancy of HIV patients, 214
Lin, Tai-Shun, 192
lipodystrophy, 144–45
Lisziewicz, Julianna
conflict with Jessen, 186
and drug trials, 191–92
and gene therapy strategies, 149–52, 200
Lisziewicz, Julianna
and Hahn’s cure, 218
and hydroxyurea research, 112–13, 189–91, 197–98
and individual genetics of HIV patients, 218
and media coverage of HIV research, 185–86
and research authorship issues, 173
and success of Hahn’s treatment, 165–73
and treatment interruption strategy, 197–98
Lori, Franco, 112, 150, 186, 189–94, 197
Lucas, 120, 130, 146, 161–62, 175
lymph nodes, 5, 163, 167, 264
lymphocytes, 24, 105, 121
lymphoma, 254
macaque monkeys, 107–9, 232–33
macrophages, 91, 130, 176
Manhattan Project, 225
maraviroc, 180
Marcus, 22–23, 262
Margolis, David, 245, 246–47
Massachusetts General Hospital, 66, 71–72, 76, 114, 187
Massie, Bob, 99, 100–101
Max Planck Institute, 149
media coverage of HIV research
and cancer research, 43, 46
and cultural significance of AZT, 57–58
and “cure” term, 51, 169–70, 185, 202, 206, 254–55
and discovery of AIDS virus, 51
and Fauci, 211
and Ho, 69–70
interviews with HIV researchers and subjects, 202
and Jessen, 185–86, 201–2, 205–6
and politics of HIV research, 60–61
and treatment interruption strategy, 195
Medicare, 213
memory T cells, 164, 168
Merck, 19, 84, 246
messenger RNA (mRNA), 149–50, 258
metastases, 53
methadone, 206–7
methionine, 109
mice, 231–35, 237, 238, 250
Michigan Cancer Foundation, 46–47, 54
Mizutani, Satoshi, 41
monocytes, 242
mortality rates of HIV, 11
mutation of HIV, 39, 58–59, 68, 93, 168, 215
naive T cells, 164
National Cancer Institute (NCI), 46, 49–50, 52, 54–56
National Institute of Allergy and Infectious Diseases (NIAID), 50, 75–76, 196, 212
National Institutes of Health (NIH), 49, 53–54, 111, 150, 236, 240, 245, 251
National Public Radio (NPR), 254–55
Nature, 74, 194
Nature Biotechnology, 250
neurological symptoms, 159–60, 214–15
neuropathy, 193
neviraprine, 241
The New England Journal of Medicine, 60, 68, 173–74, 178, 185, 186–87, 202–3, 259, 265
and drug trials, 85
New York Stock Exchange protest, 61
The New York Times, 51, 185, 211
The New York Times Magazine, 202
news. See media coverage of HIV research; specific publications
Newsweek, 69, 185–86
Nobel Prizes, 41, 225, 257
nucleotides, 47
OraQuick test, 26–27
pancreatitis, 190–91, 197–98
pandemics, 130
patents, 48–49, 58, 227–28
PCR-based tests, 15–16, 163, 241–42, 257, 263–64
peer review, 178
personalized medicine, 102
pharmaceutical industry, 198, 239–40. See also specific companies
Phase II clinical trials, 239
physical symptoms of HIV, 159–60, 213–15
Piechocka-Trocha, Alicja, 170–71
plagues, xx, 67
A Planet of Viruses (Zimmer), 35
Planned Parenthood, 45
platelets, 59
pneumoatosis cystoides intestinalis, 207
pneumocystis, 71
pneumonia, 66, 155
poliovirus, xx
politics of HIV research, 52–53, 58, 60–61, 73
polymerase chain reaction (PCR) tests, 15–16, 163, 241–42, 257, 263, 264
poster presentations, 179–80, 199–200, 211
poxviruses, 67–68, 130
privacy issues, 45, 171
prodrome, 4–5
protease enzymes, 19, 36, 69, 84
protease inhbitors, 19
Prusoff, William, 192
psoriasis, 109, 112
public health systems, 28–29, 175–76
QALYs (quality-adjusted life years), 213
radiation, 225, 244
Ragon, Terry and Susan, 269
Reader’s Digest, 46
Reagan, Ronald, 51, 53
rectal biopsies, 176, 237, 263
Rent, 57
Research Institute for Genetic and Human Therapy (RIGHT), 150
research methods, 187–88, 210
reservoirs of HIV, 200, 209, 246–48, 253–54, 265
resting T cells, 5, 165–66, 168–69, 201–2, 247, 254, 263–64
restriction enzymes, 228, 230
retrovir, 153
retroviruses, 34–40, 41, 50, 51, 257
reverse transcription, 35–36, 37, 40–41, 54, 84, 257–58
rhesus macaque monkeys, 232–33
ribonucleic acid, (RNA), 34–36, 37, 90, 149–50, 170–71, 200, 258, 265
ribosomes, 258
ribozymes, 200
Richard (HIV patient), 214
Richman, Douglas, 265
Rideout, Janet, 54–55
Roche, 18–19, 83, 84
Rosen, Craig, 73
Rosenberg, Eric, 187
rous sarcoma virus, 41
Saez-Cirion, Asier, 255–56
safety issues, xv, 56, 237–38
same-sex marriage, 57
Sangamo BioSciences, 227–29, 235, 249, 250, 259, 270
Sanger, Margaret, 45
saquinavir, 19, 84
Sarnoff, David, 46
Schoofs, Mark, 202, 203
Schooley, Chip, 72, 76
Schwartz, Lisa, 102, 106, 269
Schwartz, Mark, 102, 106, 269
Schwarzenegger, Arnold, 251
Science, 72, 165, 265–66
selection pressures, 237, 238
self-esteem of HIV patients, 143–44
serine, 109
severe combined immunodeficiency (SCID), 62, 235
Shilts, Randy, 31
short hairpin RNA, 200
sickle-cell disease, 112
side effects of drugs
author’s experience with, xvii–xviii
and AZT, 98
and bone marrow, 59
and Brown, 98
drug toxicity, 157, 189–91, 193, 197–98
and early treatment approach, 136
and hydroxyurea trials, 191
long-term effects of, 139
variety of responses to HIV drugs, 87
signaling molecules, 223
Siliciano, Bob, 5–6, 165–66, 168, 201
simian immunodeficiency virus (SIV), 38–39, 232–33
SIVmac, 232–33
slow progressors, 217–18
small interfering RNAs (siRNAs), 258
smallpox, 67–68, 130
social impact of HIV, 144–46
Sodroski, Joe, 73–74
Somatix, 227, 228
A Song in the Night (Massie), 99
Sontag, Susan, 67
Spanish flu, 38
spleen, 225
“Star Wars” approach to HIV treatment, 227, 231. See also CCR5 protein and receptor; zinc finger nucleases (ZFNs)
statistical analysis, 134, 213, 233
stem cells
and author’s research background, xiv
and bone marrow transplants, 225
and cancer treatments, 121–24
and conditioning regimes, 201
and cord blood, 123–24, 243–44
and gene therapy strategies, 157–61, 177, 179–80, 201, 203, 217, 253
and humanized mouse model, 234
and ongoing HIV research, 259
and zinc finger nucleases, 249–51
sterilizing cures, xix, 267
steroids, 223
stigma associated with HIV, 40, 43, 57, 67–68, 75, 141, 144–45
storm trooper (killer) T cells
and acute lymphoblastic leukemia, 221
and elite controllers, 99
and HIV infection process, 88, 91
and HIV tests, 170
and human leukocyte antigen, 74–75, 81, 82
and immune response in elite controllers, 108
and immunology research, 72–74
and ongoing HIV research, 256
and success of Hahn’s treatment, 163–64, 169–70, 172
and treatment interruption strategy, 79–80
Strategic Management of Antiretroviral Therapy (SMART), 196
super strain HIV, xiv–xv
Switzerland, 70
symptoms of HIV infection, 4–6, 14, 40, 50, 91–92
T cells
and bone marrow transplants, 157–58
and destruction of CCR5, 231
and elite controllers, 76–77, 100–101
and gene therapy strategies, 222–23
grown in the lab, 226
helper T cells, 80
and HIV infection process, 68–69, 88, 91
and the immune response, 24, 187–88
and long-term consequences of HIV infection, 215
memory T cells, 164, 168
naive T cells, 164
resting T cells, 5, 165–66, 168–69, 201–2, 247, 254, 263–64
T cell receptor (TCR), 81, 82, 223
and treatment interruption strategy, 79–80, 194–95
and viral latency, 248
and viral replication process, 5
See also commander T cells; storm trooper (killer) T cells
TAR decoy molecule, 200
Temin, Howard, 40–41
testing for HIV. See diagnosis of HIV
Thiel, Eckhard, 128, 178
Thomas, E. Donna, 225
3TC (lamivudune), 241
thymidine, 47–48, 192
thymus, 24, 234
Time, 16, 69–70
Timothy Ray Brown Foundation, 269
To Kill a Mockingbird (Lee), 117
transcription, 34, 37. See also reverse transcription
transmission of HIV, xvi, 51–52
treatment interruption strategy (drug holidays), 77, 187, 189–90, 194–98, 237
Trenton patient, 238
Tresor, 21, 22
tryptophan, 109
tuberculosis, 68
tumors, 53
tumor-suppression gene, 246
uracil, 34
U.S. Congress, 46
U.S. Department of Health and Human Services, 51
U.S. Food and Drug Administration (FDA), 58–60, 103, 112, 191, 248
uterine cancer, 45
vaccines, 67–68, 79, 231, 233
valproic acid, 246
varicella zoster, xx
Vietnam War, 224
viral antigens, 81
viral envelope, 36, 37, 90
viral enzymes, 36, 37
viral latency, 5–6, 200, 246–48, 254
viral load, 157–58, 168–69, 235
viral replication process, 5
viremic controllers. See elite controllers
virions, 36
VISCONTI cohort, 256
vorinostat, 246, 247, 248
Wagner, John, 244
Walker, Bruce
background, 70–71
and the Conference on Retroviruses and Opportunistic Infections, 180
and drug trials, 193–95
and early HIV research, 72–77
and early treatment approach, 186–88
and elite controllers, 99–102, 103, 106
and funding for HIV research, 269
and gene therapy strategies, 169–71, 173
and genetic research, 123
and the International AIDS Conference, 79
and killer T cell research, 82
and media coverage of HIV research, 202
and ongoing HIV research, 266, 268–69
poster presentation, 180
and research authorship issues, 173
and research ethics, 188
and success of Hahn’s treatment, 169–70
and treatment interruption strategy, 194–97
The Wall Street Journal, 202
Walter Reed National Military Medical Center, 225
wasting syndrome, 40
welfare systems, 175–76, 261–62
Wellcome, 54–56. See also Burroughs Wellcome Company
White, Ryan, 52
white blood cells, 88, 156, 167. See also immune system
Whitehead, Emily (“Emma”), 221–24
Woodward, Mary, 44–45
World AIDS Institute, 269
World Health Organization (WHO), 67–68
yellow fever, 38
Yersina pestis, 130
Yukl, Steve, 264, 265–66
Zaia, John, 200–201, 222, 250
Zerit, 192, 193
Zimmer, Carl, 35
zinc finger nucleases (ZFNs), 228–31, 230, 235–38, 249–50
ZKRD bone marrow database, 129
