Chapter 9
Nanochemistry
Nanochemistry involves the synthesis of molecular nanostructures measuring 1–100 nm. These could serve as molecular components for nanorobots and other molecular devices that could be used in medicine, analysis, synthesis, electronics, data storage, or material science. One current research goal is to design a molecular computer. Current computers use silicon integrated circuits, but there is a limit to how small these components can be made. Designing electronic devices and computers that operate at the molecular level will allow a dramatic reduction in scale and a corresponding increase in computer power. In order to make these dreams a reality, it is necessary to design nanostructures that are the molecular equivalents of wires, switches, data storage systems, and motors.
Carbon allotropes
Allotropes are ordered structures consisting entirely of one type of atom. Diamond is a carbon allotrope where each carbon atom is covalently linked to four other carbon atoms to form a very strong lattice (Figure 104a). Because of its strength, diamond has industrial applications such as diamond-tipped mining drills. It is one of the hardest and most chemically inert materials known, and it also has useful optical properties.
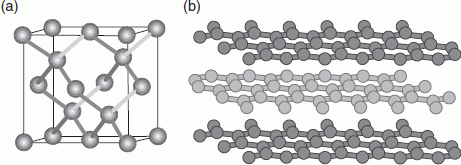
104. Structures of (a) diamond and (b) graphite.
Graphite is another carbon allotrope where the atoms are arranged in layers of planar, aromatic rings (Figure 104b). Each layer involves strong covalent bonds, but only weak intermolecular interactions exist between each layer. This allows the layers to ‘slide’, which makes graphite ideal as the ‘lead’ for pencils. For the same reason, graphite is used as a dry lubricant in machinery and engines. Surprisingly, graphite has been detected in hip transplants, where it seems to lubricate the metal–metal contact of the hip joint. It is not yet known how this graphite is generated, but it is possible that the hip implant itself ‘grinds up’ proteins to form carbon, which is then converted to graphite.
Graphite also conducts electricity because of the relatively mobile π electrons present in aromatic rings. A recent innovation that makes use of graphite’s conductivity involves the attachment of three different enzymes to graphite beads. One of the enzymes catalyses the splitting of hydrogen gas to form two protons and two electrons. Because of graphite’s electrical conductivity, the electrons shuttle through the bead to a second enzyme which catalyses the reduction of a substrate. The product from that reaction undergoes a further reaction catalysed by the third enzyme. This system can be viewed as a miniature chemical factory and gained an Emerging Technology Award from the Royal Society of Chemistry in 2013.
A single layer of graphite is called graphene, and was first produced in 2004 at the University of Manchester, earning its inventors the 2010 Nobel Prize in Physics. As well as conducting electricity, graphene is the thinnest, strongest material known to science with a tensile strength 300 times greater than steel. It is also stable to heat, and relatively inert to chemicals.
Because of these properties, graphene has many potential applications as a component in chemical sensors, medical devices, solar cells, hydrogen fuel cells, batteries, flexible displays, and electrical devices. One potential use for graphene is as a desalination filter. The idea would be to punch pores into the graphene that would allow water to squeeze through, but not salts. Another possible application for graphene is as an alternative to Kevlar in body armour. In the field of sensors, it is thought that a graphene-based device might be capable of detecting bacterial infections or contamination.
At present, much of the work carried out on graphene has been in the research lab, and the next stage is to apply that research to create new materials in the factory—a process that may take 20–40 years. One practical problem is devising an economical method of producing graphene on a large scale. This is essential if it is to be used commercially.
Fullerenes are a third type of carbon allotrope, involving spheres or cage-like structures. The carbon atoms are arranged in hexagonal and pentagonal rings, the latter introducing the curvature required to form a sphere. The best-known example of a fullerene is buckminsterfullerene  (or fullerene-60), which has a pattern and shape akin to a soccer ball (Figure 105)—the number 60 refers to the number of carbon atoms present in the structure.
(or fullerene-60), which has a pattern and shape akin to a soccer ball (Figure 105)—the number 60 refers to the number of carbon atoms present in the structure.
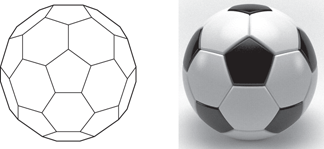
105. Arrangement of carbon atoms in fullerenes.
Fullerene-60 was discovered from experiments that were designed to mimic what kind of chemical reactions might be taking place in outer space. In 2010, an infrared telescope established that fullerene-60 was indeed present in interstellar gas clouds. However, C60 structures are also formed much closer to home—near candle flames! It is thought that the process starts with the formation of small carbon cages which progressively increase in size by swallowing up atoms of vapourized carbon. However, it is not yet understood how the small cage fullerenes are formed in the first place. Fullerenes of different cage sizes include C28, C32, C50, and C70. Unlike C60, these are not perfectly spherical. For example, fullerene-70 is shaped like a sausage. The discovery of buckyballs earned Henry Kroto the Nobel Prize.
To date, fullerenes have not been put to any commercial use. However, there are plenty of suggestions for future applications. One suggestion is to use them as drug-delivery vehicles to introduce drugs or genes into cells. Other potential applications include lubricants, electrical conductors, solar cells, and even safety goggles. A modified fullerene has been used in the synthesis of a molecule that can respond to weak magnetic fields (Figure 79), and fullerenes have also been used as wheels in a nanocar (Figure 117).

117. A nanocar.
Nanotubes
Carbon nanotubes are molecular cylinders made up of carbon atoms. The walls of the nanotube are made up of hexagonal rings (Figure 106), and are essentially a rolled up layer of graphene. Each end of the nanotube is fullerene in nature and contains pentagonal rings that introduce the curvature that seals off the tube. Their diameter is about 1 nm (about the same diameter as a strand of DNA), and their length can be up to 132,000,000 nm.
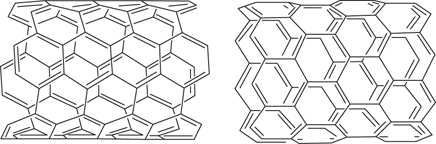
106. Structural variation in nanotubes.
The properties of nanotubes vary depending on their dimensions and atomic arrangements. Different lengths and diameters result in different electronic properties, which make nanotubes useful in nanoelectronic circuitry as insulators, semiconductors, or conductors.
The relative orientation of the rings making up the walls of a nanotube has a profound effect on its electronic properties. Hence, nanotube A in Figure 106 is a semiconductor, whereas nanotube B is a full conductor. The price of nanotubes is dropping dramatically and it is anticipated that they will have a widespread use in electronics by 2020. The field of nanoelectronics could well result in future molecular computers.
Nanotubes have been shown to be stronger than steel, but at a sixth of the weight, making them extremely useful in materials science. The strength to weight ratio of nanotubes gives them potential applications in aircraft parts, car parts, and sports equipment, especially if the nanotubes are ‘bundled up’ to form fibres with high tensile strength. Their large surface area is also useful. For example, they could be linked to enzymes and used for synthesis or in hydrogen fuel cells.
Nanotubes can be single-walled or multi-walled. Multi-walled nanotubes are multiple rolled layers of graphene and have improved chemical resistance. This is important when linking molecules to the surface of nanotubes, since the linking process can punch holes in the nanotube wall and affect its mechanical and electrical properties. With a double-layered nanotube, only the outer layer will be affected. Nanotubes have been modified to bind organic molecules capable of detecting other molecules, which make them useful components in sensors or bioelectronic ‘noses’ for monitoring food quality, or detecting explosives and chemical leaks. Alternatively, nanotubes linked to light-sensitive molecules could be useful in the design of solar cells and energy storage.
Nanotubes might also be useful as capsules for other molecules. Nature has already achieved this. The tobacco mosaic virus consists of a nanotube made up of identical viral proteins. The proteins self-assemble to form the nanotube and encapsulate viral RNA. Some researchers are looking into designing self-assembling nanotubes that will hold buckyballs, since it is believed that these will have good electronic properties.
Rotaxanes
A rotaxane is a nanostructure where two interlocking molecules form the equivalent of an axle and a wheel (Figure 107). The molecule representing the wheel is a large cyclic structure (macrocycle), while the molecule acting as the axle is dumbell-shaped. The two bulky groups at either end of the axle prevent the macrocycle ‘slipping off’ the axle. The macrocycle can either rotate around the axle or move along its length from one end to the other. However, the latter movement is not a smooth process since the axle contains one or more ‘docking’ sites that temporarily hold the macrocycle in position. This is known as a molecular shuttle. The interactions are strong enough to ensure that the macrocycle spends most of its time interacting with the docking sites available, but weak enough to allow the macrocycle to shuttle between the available sites.
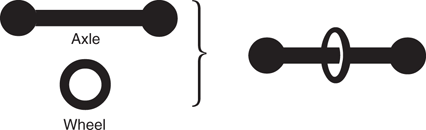
107. General structure of a rotaxane involving a dumbell-shaped molecule ‘threaded’ through a macrocycle.
One example of a molecular shuttle is shown in Figure 108. The axle has two docking stations involving aromatic rings, and bulky silicon groups at each end to prevent the ‘wheel’ slipping off the axle. The aromatic rings at each docking site can interact with the aromatic rings of the macrocycle—a form of interaction known as a π–π interaction. The interaction is relatively weak and so it is possible for the wheel to shuttle between both docking sites. However, in this example there is a preference for the wheel to bind to the right-hand docking station. That is because the docking stations are not identical. One of the docking stations has oxygen atoms attached to the aromatic rings, while the other has nitrogen atoms. The latter site interacts more strongly with the ‘wheel’, and so the wheel spends 84 per cent of its time bound at that site, and the remaining 16 per cent bound at the other docking site.

108. Example of a rotaxane acting as a molecular shuttle.
This preference can be altered. Under acidic conditions, the nitrogen atoms attached to the right-hand docking site become protonated and gain a positive charge. Since the wheel already contains positively charged nitrogen atoms, it is repelled from the right-hand docking site and binds exclusively to the left-hand docking site. Therefore, the rotaxane acts like a molecular switch. It is not a perfect switch, since there should be exclusive docking to different docking sites under different conditions. Nevertheless, the example illustrates the potential of rotaxanes as molecular switches.
Molecular switches can have a number of applications. For example, a research team at Edinburgh University designed a rotaxane that acted as a ‘switchable’ catalyst for organic synthesis (Figure 109). The nitrogen atom at the centre of the axle is responsible for catalytic activity. Under basic conditions, the wheel binds to either of the two docking sites leaving the nitrogen atom free to act as a catalyst. Under acid conditions, the nitrogen becomes protonated and gains a positive charge, making it a stronger docking site for the wheel. The wheel now moves to the centre of the axle and conceals the catalytic site. In this example, the wheel contains oxygen atoms, which form strong hydrogen bonds with the protonated amine (Figure 110).
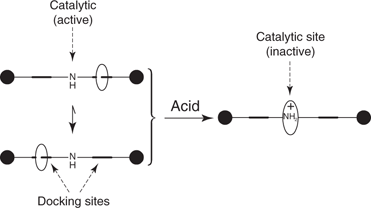
109. A rotaxane acting as a switchable catalyst.
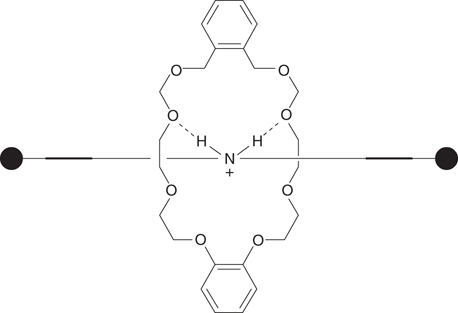
110. Hydrogen bonding interactions between the wheel and the protonated amine on the axle.
More recently, a rotaxane has been designed with two docking sites capable of catalysing two different kinds of reaction. The ring binds to one of the catalytic docking sites under acid conditions, and binds to the other catalytic site under basic conditions. Therefore, the same rotaxane can be used to catalyse two different kinds of reaction depending on the reaction conditions used.
Another rotaxane was designed to synthesize a tripeptide (Figure 111). The axle had three amino acids attached, and the wheel was threaded on to one end of the axle. As the wheel moved along the axle, it picked up the amino acids one by one in the order presented. There was no blocking group at the other end of the rotaxane and so the wheel with the attached tripeptide dropped off when it reached the end. The tripeptide could then be cleaved from the wheel. This research demonstrates that it is possible to design molecular synthetic machines that automatically produce new molecules, but there is a long way to go before this approach can compete with conventional synthesis.
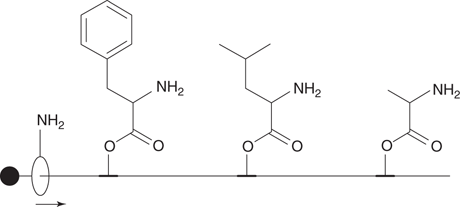
111. A rotaxane acting as a molecular synthetic machine.
Rotaxanes with an axle made up of alkyne functional groups have been synthesized at Oxford University (Figure 112). Since alkynes are linear, the axle is linear and contains only carbon atoms. Such rotaxanes have been proposed as potential molecular wires for nanoelectronics. The wheel would act as an insulator as it shuttles back and forth.
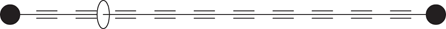
112. Rotaxanes containing linear alkyne groups.
Another approach towards molecular wires is to prepare polyrotaxanes that contain several wheels on the central axle (Figure 113). When several wheels are present on the one axle, they interact with each other, and this serves to stiffen and straighten the rotaxane. The efficiency with which electrons travel along molecular wires is faster with rigid rotaxanes.
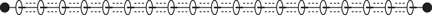
113. Polyrotaxanes designed to have interactions between the wheels.
If rotaxanes are to prove useful as switches or wires, they will have to be connected and integrated into rigid structures. One approach is to incorporate rotaxanes into the structure of metal-organic frameworks, such that the moving parts of each rotaxane is located within a pore. If this proves successful, then it opens up the possibility of creating solid-state molecular switches or machines.
Rotaxanes have also been used to create molecular ‘muscles’ that contract or expand under different stimuli. This involves two interlinked rotaxanes, where the end of each axle is covalently linked to the wheel on the other axle (Figure 114). This has been dubbed a daisy chain rotaxane. The lengths of the extended and contracted forms are 4.8 nm to 3.6 nm respectively. By polymerizing these daisy chain rotaxanes into molecular ‘fibres’, the resulting contractions and expansions are magnified (Figure 115). A French research team has linked together 3,000 rotaxanes that contract from 15.8 micrometres to 9.4 micrometres. The polymerization of the rotaxanes was achieved by using blocking groups that bind to a metal ion. Metal ions then act as a molecular ‘glue’ to hold the daisy chain rotaxanes together. The next challenge will be to bundle these fibres together.
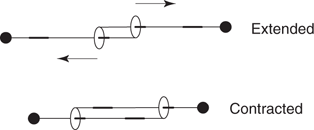
114. Linked rotaxanes that mimic muscle action.
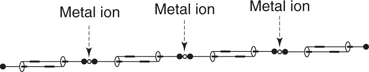
115. Polymerized daisy-chain rotaxanes into a molecular muscle fibre.
Nanoparticles
Nanoparticles are approximately 1–100 nm in size. Their properties are distinct from larger scale materials because of their size and relatively large surface area, and they have a wide range of actual and potential applications in medicine, manufacturing, materials, energy, and electronics. For example, it is possible to synthesize spherical nanoparticles that encapsulate a drug or DNA, then administer them to deliver their load to a patient’s cells. For example, a lipid nanoparticle carrying the anti-cancer drug paclitaxel (Taxol) is currently undergoing clinical trials. Nanoparticles are also being designed that combine diagnostics with therapeutics (theranostics). For example, a nanoparticle has been designed to identify tumour cells. On binding, it breaks open to release an anti-cancer drug that treats the cancer, along with a dye that reveals where the tumour is.
Nanodelivery systems can also be used to protect neutraceuticals (e.g. vitamins) from the destructive effects of stomach acids. Nanocapsules have been constructed from proteins and sugars that are naturally found in food. The nanocapsules are stable to stomach acids, but are broken down by enzymes in the intestines to release the neutraceutical. Nanocapsules loaded with vitamin D could be added to soft drinks to prevent rickets.
Nanoparticles have uses in medicine, other than drug delivery. For example, nanoparticles have been developed that could potentially stop internal bleeding resulting from road accidents or terrorist bombs. The nanoparticles are designed to stick to activated platelets and speed up clot formation, thus reducing the chances of a patient bleeding to death. So far, the technique has only been tested on animals.
It has been discovered that carbon nanoparticles prevent the development of mosquito larvae, and so they could be useful in controlling malaria. The nanoparticles have a long lifetime, which would be an advantage in terms of their insecticidal activity, but could be a potential disadvantage if they have unforeseen environmental or ecological effects.
Nanotechnology and DNA
Nanostructures constructed from DNA have many potential applications. DNA is nature’s data storage molecule and carries the codes required for an organism’s proteins. In addition, its structure allows that information to be copied from one generation to another. The nucleic acid bases (ATGC) are the genetic alphabet, and a molecular recognition process takes place such that the base pairs are always A–T or G–C. This is crucial to the double helical structure of DNA, as well as the 3D shapes of RNA molecules.
Scientists have now taken advantage of base pairing to synthesize single-strand DNA molecules that self-assemble into predictable shapes determined by the sequence of bases present. For example, if a DNA strand contains complementary base sequences at different parts of the strand, then the molecule can coil up to allow base pairing (Figure 116). Using this approach, scientists have created 2D pictures using DNA, as well as 3D shapes. This is a process known as DNA origami.
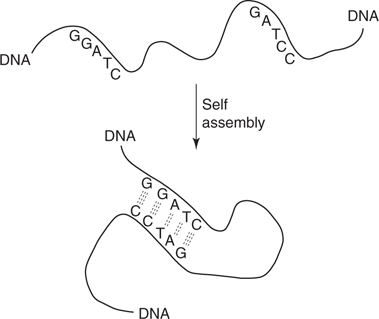
116. DNA origami.
This approach has been used to construct DNA nanorobots that can carry out robotic tasks such as sensing, computation, and cell-targeting. One research group has created a barrel-shaped DNA robot that is 35 nm in diameter and 45 nm long. The structure includes a hinge that allows the barrel to open up like a clam. Short DNA strands are present to keep the barrel closed until the nanorobot encounters an antigen that interacts with the DNA strands. This unlocks the barrel, which can then open to release its contents. So far, the nanorobot has only been tested on cell cultures, but it has the potential to carry drugs or antibodies to specific sites in the body. A similar idea for drug delivery involves DNA cubes that are designed to ‘unzip’ when they interact with RNA molecules that are unique to prostate cancer cells.
A DNA ‘walker’ has been designed that responds to light and can follow a trail along a surface. The trail consists of a series of ‘poles’ represented by strands of DNA, with each pole having a long section and a short section. The walker has two DNA legs—one short and one long. The walker binds to the first pole with its long leg binding to the long section of the pole and its short leg binding to the short section of the pole. When light is shone on the surface, the short section of the pole is split from the long section and floats away. The short leg of the walker is now free to seek out the short section of the next pole and bind to it. When it succeeds, it pulls the long leg along with it. In theory, this system could be used to design a nanolaboratory, where the walker picks up building blocks from different poles and combines them to form a product.
Examples of nanodevices and nanomachines
Nanodevices are being designed that mimic instruments or machines at a molecular level. For example, a nanodevice the size of a memory stick has been designed that can sequence DNA. The device makes use of two proteins. One of the proteins is a genetically modified form of a natural protein called α-hemolysin. This protein contains a pore and is embedded in a membrane-like surface, such that a nanopore is created through the membrane. The second protein can bind DNA and is linked to the outer surface of the pore protein. When it binds DNA it feeds it through the nanopore. As the DNA is threaded through the pore, the flow of ions through the pore varies depending on which base is in transit. The variation in ion flow can be measured and allows the DNA to be sequenced. Currently, the instrument can sequence up to 48,000 bases. A similar approach could potentially sequence proteins.
An all-carbon photovoltaic cell has been produced that involves carbon nanotubes, fullerenes, and graphene. The carbon nanotubes act as the light absorber and electron donor, while fullerene-60 buckyballs act as the electron acceptor. These are sandwiched between an anode of reduced graphene oxide and a cathode of more carbon nanotubes. The efficiency of the cell is too low to be commercially useful, but the technology could be incorporated into current solar cells to make them cheaper and more efficient.
A number of research teams have been involved in what might seem rather unusual projects, such as the design of molecular motorboats, cars, and trains. These may seem no more than curiosities, but the knowledge gained from such projects could eventually lead to commercially useful nanomachines. An example of a nanocar was synthesized in 2005 (Figure 117). The wheels are fullerenes, and a rigid molecule constructed from straight chain aromatic rings and alkyne groups serves as the chassis. In truth, this contraption is better described as a nanocart, since there is no molecular motor present to propel it. However, research teams are working on that! The contraption can roll across a surface because the bonds linking the buckyball wheels to the chassis are rotatable.
Nanotechnology: safety and toxicology
Nanotechnology is already used in coatings, textiles, food, cosmetics, and medicine, and is certain to have a major influence on future society. There are many potential applications, but it is important to carry out rigorous safety and toxicology tests on nanomaterials before they are introduced on a large scale. For example, what effect do they have on human health if they are inhaled, swallowed, or absorbed through the skin? Could they irritate the lungs and cause damage similar to the effects of breathing in fine dust? What effect might nanoparticles have on the human immune system? If large quantities of nanoparticles enter the environment, how would that affect insects, birds, fish, and animals? Finally, how might nanotechnology be misused by criminals, terrorists, and unscrupulous institutions?
These questions have already been raised, and so properly designed tests need to be carried out to assess if there are any risks. Unfortunately, many of the toxicological studies carried out so far have been flawed because of the excessive quantity of material tested. Proper toxicology testing should establish whether a material is safe under realistic conditions and concentrations. For example, table salt can be shown to be toxic in high doses, but nobody would seriously consider removing it from supermarket shelves. To that end, there has been some discussion about introducing a regulatory system that would oversee nanotechnology. The EU has already issued guidelines (in 2011) on how toxicology tests should be carried out on nanoparticles.