
9
Surgeons and Risk
You may remember that, in Chapter One, we looked briefly into the history of heart surgery. One of the distinguishing features of this specialty was that it lagged behind other medical and surgical specialties because it had to wait until, among other things, the heart–lung machine was invented. Without this vital piece of equipment, the only heart operations that could be carried out were those that could be completed in a few minutes, before the brain died through lack of oxygen. This did not stop aspiring and innovative heart surgeons in the first half of the 20th century from having a go, and a few such operations were actually carried out by these early pioneers. Two in particular serve to illustrate the kind of person who would be a heart surgeon in those days of yore.
The first operation is a ‘closed mitral valvotomy’. The mitral valve is a delicate structure that sits in an inaccessible place in the middle of the left side of the heart. The job of the mitral valve is to let blood in from the left atrium (a collecting chamber) to the left ventricle (the main pumping chamber of the heart). When it does its job properly, the mitral valve opens widely to allow blood from the atrium to flow into the ventricle as the ventricle relaxes to fill up, ready for the next heartbeat. When the ventricle begins to beat in order to pump the blood to the body, the valve shuts tight to prevent back-flow, so that all the blood is pumped forward into the aorta to supply the body with oxygen and goodness.
The mitral valve is vulnerable in rheumatic fever, a medical condition that tends to happen in childhood, and begins with a simple sore throat caused by a fairly common bug from a family of bacteria called streptococcus. The body’s immune system reacts to this streptococcus infection by mounting a vigorous defence, sending white cells and antibodies to destroy the alien invader. Unfortunately, the powerful immune response to this particular streptococcus is not very selective, and sometimes attacks the body’s own tissues, especially the joints (hence the ‘rheumatic’ part of the fever), but also, incomprehensibly, the heart valves, including the mitral valve. As a result of this attack, the valve becomes horribly inflamed and, later, scarred as the body tries to repair the damage. As the years pass, this scarring progressively becomes thicker and harder. The leaflets of the valve, normally gossamer-thin and flexible, become thick, unwieldy, and calcified. Finally, the leaflets end up fused into an ugly, craggy white rock with a tiny slit in the middle. Through this slit, five litres of blood are somehow supposed to pass every minute if the patient is to stay alive, and, not surprisingly, this is an untenable situation. Gradually, pressure rises upstream of this narrowed valve. This high pressure in the left atrium backs up into the lungs and into the right side of the heart, the veins of the body, and the liver, all of which are upstream of the valve. The lungs begin to leak and the right heart fails, the liver and legs swell up, and the patient becomes tired, very breathless, and in a poor shape indeed. The only way to relieve the symptoms is by having the mitral valve open properly again, but, as it is deep within the heart, fixing it without a heart–lung machine is virtually impossible.
Virtually impossible is not, however, impossible. Pioneering heart surgeons actually found a way before the invention of the heart–lung machine, and that operation is closed mitral valvotomy. Here is the recipe, but please do not try this at home:
Henry Souttar was the first surgeon to carry this out successfully in London, as far back as 1925, but it was probably an American in Philadelphia, Charles Bailey, who convinced the world that there was some merit in this apparently crazy, desperate procedure. Bailey first tried out mitral valvotomy on four patients, and killed every single one of them. As a result, he was banned from operating in three hospitals in the Philadelphia area. Undaunted, in 1948, he decided to have another go. He planned to operate on two more patients on the same day, but in two different hospitals. His rationale for this strategy was most disturbing. He figured out that if the first patient died at one hospital, he could rush across town and get the second operation started at the other hospital before the news broke out and he was banned from operating at these hospitals as well. The morning patient died on the operating table, but the afternoon patient survived, and the operation became established. As you have probably surmised, this is hairy surgery. It requires supreme confidence, nerves of steel, and fast decision-making, but, compared to the next operation I shall tell you about, closed mitral valvotomy is a walk in the park.
The condition of atrial septal defect, or ASD, is a relatively common congenital abnormality. In this, the baby is born with a hole between the right atrium and the left atrium, which are just upstream of the right and left ventricles. The two atria should not communicate at all, as they are naturally separated by a wall, which is called the atrial septum. There is a good reason for this separation, and it is easy to understand. This is how blood circulates through the heart:
Having been around the body to deliver oxygen and food, the blue and useless oxygen-poor blood comes back to the heart and lungs to be loaded with oxygen and pumped around the body again. This is its journey:
So, to simplify the journey, it goes like this:

When a hole exists in the wall or septum between the atria, the blood flow changes to this:
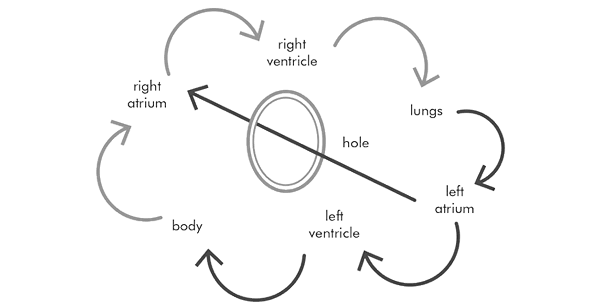
As you can see, a lot of the blood ends up going round in circles: right atrium, right ventricle, lungs, right atrium, right ventricle, lungs, and so on. This is a terrible waste of the heart’s pumping energy. The right ventricle ends up having to pump twice or three times as much blood just to keep the flow going around the body at an acceptable level. Not being designed to do this, the right ventricle fails. As for the lungs, they end up handling twice or three times as much blood going through them as necessary, their blood pressure rises, and they eventually fail.
Babies who are born with an ASD appear perfectly normal to begin with, and usually have a normal early childhood, but their right ventricles and lungs are overworking every minute of the day and night. Gradually, the right ventricle and the lungs begin to fail, and, sometime in childhood, adolescence, or young adulthood, patients with ASD become short of breath, and that is when they come to the attention of doctors. The doctors examine the patients, do a few tests, and the hole is found.
Closing an ASD is no great surgical challenge. It’s only a hole, after all, and it takes a competent surgeon no more than about five to 20 minutes with a stitch to cobble it up or patch it, but, in order to reach it, you have to open the right atrium. Do this without a heart–lung machine and without isolating the heart from the circulation, and the patient’s lifeblood ends up on the floor.
Before the heart–lung machine was invented, the pioneering heart surgeons of the 1930s and 1940s tried a variety of methods to get at the hole ‘blind’, without actually opening the atrium to see it. They finger-pushed the wall of the atrium into the hole and tried to cobble it from outside ‘by feel’. They tried to insert plugs and other devices to block the hole. They developed many ingenious methods, some of which worked partially, and some of which did not work at all, but all of them had an appalling mortality rate. Then someone had the great idea of clamping the two huge veins that pour into the right atrium, opening it, and closing the hole very, very quickly. The reason for this need for speed is that, during that time, there is no circulation, and the brain and the patient are both rapidly dying. As we know, the brain will only survive the absence of circulation for a few minutes. To help prolong the period of zero-circulation that the patient will tolerate without brain damage, it seemed a good idea to cool the body and the brain before the circulation is stopped.
Finally, on 2 September 1952, in Minnesota, USA, surgeon John Lewis made this operation a reality. Together with three assistants (Richard Varco, Mansur Taufic, and Walton Lillehei), Lewis operated on a five-year-old girl with an ASD. They put the little girl to sleep and cooled her from normal body temperature of 37°C to 28°C with refrigerated blankets. They then opened the chest, and clamped the great veins that flow into the right atrium. They opened the right atrium, sucked out the blood that was in it, quickly stitched up the hole, hastily closed the right atrium, and took off the clamps. The heart started pumping again. They re-warmed the girl, and she survived, without brain damage, after a period of zero circulation that lasted only five and a half minutes. The girl was cured of her ASD, and her operation was the world’s first successful procedure on the open human heart under direct vision. This marked the beginning of the era of open-heart surgery. A couple of years later, the heart–lung machine was invented, and heart surgery took off.
When John Lewis died in 1993, his fellow surgeon during that seminal operation Walton Lillehei said ‘From that memorable beginning in Minneapolis, open-heart surgery has grown to be one of the major medical contributions of the 20th century. In the past year, worldwide, more than one open-heart operation was done every minute! Such was the legacy that John left.’
Interestingly, Lillehei went on to pioneer many innovations in heart surgery, the most notable of which was the use of a human being as a heart–lung machine! He operated on many children by using one of their parents as a machine to keep the child’s circulation going. Both parent and child were anaesthetised, and tubes attached to connect the child to the mother or father’s circulation, as though the child was an extra ‘organ’ of the parent. Under these circumstances, the child’s heart could be isolated from the circulation and fixed, while the parent’s heart, lungs, and circulation kept the child alive. This type of operation must be one of the very few that can actually have a 200 per cent mortality rate, in that it can kill the patient and the mother or father of the patient (and occasionally, it did).
I have segued into the early milestones of heart surgery for a good reason, and that is to illustrate clearly that the very nature of the specialty in those days attracted a very particular type of surgeon: an adventurer, a risk-taker, a daredevil, a pioneering, determined, ruthless, and courageous sort of person with no room for lengthy introspection or nagging self-doubt. These were almost universally the attributes of the pioneering cardiac surgeon’s personality, and some of these traits still remain in evidence in some heart surgeons to this day.
So what do we know about this side of surgeons’ personality characteristics? And more specifically, does it have an impact on their results, which are, after all, what we care about?
Psychologists are fond of describing and classifying people according to various psychological and personality traits. In a way, the father of medicine, Hippocrates, was something of a personality psychologist. He believed that the body fluids, or ‘humours’, were responsible for our psychological make-up. Blood makes us animated and confident (sanguine), phlegm causes us to be cool and detached (phlegmatic), yellow bile makes us bitter and twisted (jaundiced), and black bile causes us to be depressed and miserable (melancholic). Modern-day psychology recognises these and many other personality types and personality traits. Some of these are well known to the layperson, such as extrovert versus introvert, analytical versus intuitive, and so on. Of course, many different aspects of personality can be studied and assessed. More recently, there has been a heightened interest in the particular trait of personality that determines how willing an individual is to take risk. This is known as ‘risk propensity’, and much of the interest in it has arisen as a result of the impact it can have on decisions made in the financial and managerial worlds, where it is self-evident that excessive risk takers, such as rogue traders or bankers, may lead their organisations into financial ruin.
But risk propensity plays a major part in surgery and its outcomes, too — not least in heart surgery. We have seen in this chapter that pioneering heart surgeons were gung-ho risk-taking daredevils. They had to be: without taking at least some risk, no progress would have been possible. One who is petrified by fire will never blaze a trail.*
[* We should pause briefly to pay tribute to those patients who willingly submitted themselves to these trailblazing procedures. Whereas surgeons undoubtedly took risks with their careers, futures, and standing within the medical community, their patients took the ultimate and far greater risk: they gambled with their lives. They fully deserve our admiration and respect.]
I suspected that this risk-taking culture could be seen in the personality traits and behaviour of some surgeons more than others. If it could be seen, it could be measured. So I decided to ask the heart surgeons at Papworth Hospital to locate themselves and their fellow surgeons on a scale representing the range of risk propensity. I did this by distributing a survey that asked participants to use a visual analogue scale that ranged from one extreme (no risk-taking whatsoever, whatever the situation) to another (taking crazy risks all the time).
Here is the visual analogue scale as presented in the survey:
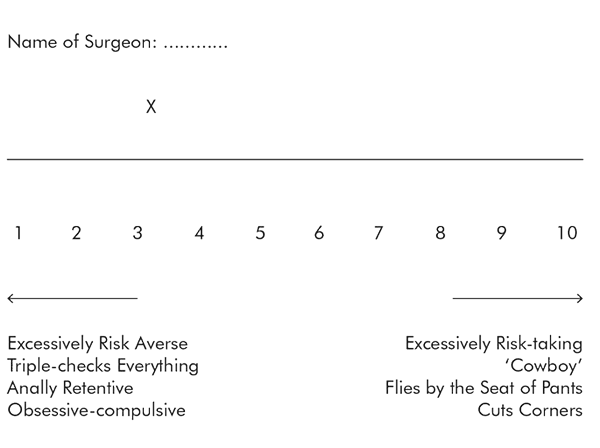
Participants were asked to answer the question for every surgeon at Papworth by placing an ‘X’ to indicate where each surgeon fell, in their opinion, on the risk-propensity scale. Both extremes of the scale were made to look equally unpleasant and negative, so that surgeons would not be placed towards the ends unless they were truly perceived to be deviant. All the surgeons themselves, as well as a group of anaesthetists and assistants who worked very closely with them, filled in the survey, so that, in total, I had 29 responses about the 13 senior surgeons who work at my hospital. This is what the survey showed:
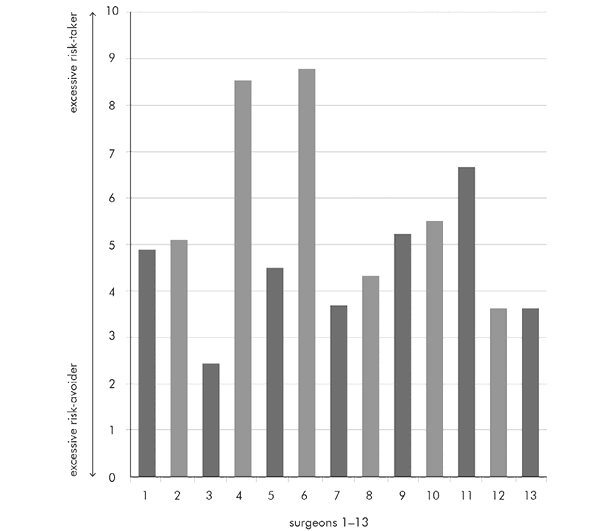
As you can see, there are marked differences. We have two or perhaps three surgeons who can most assuredly be described as risk-taking cowboys, and one who is firmly ensconced in a mouse hole at the obsessive-compulsive opposite end of the scale. In a way, this is not at all surprising. Like all human beings, surgeons differ in many aspects of their personality traits, and risk propensity is simply yet another personality trait. The question that immediately springs to mind is which of these, if any, is associated with better clinical outcomes. The quickest way of doing this is to look at risk-adjusted mortality ratios, or RAMR. This is simply the ratio between expected mortality and actual mortality, expressed as a decimal number. If the ratio is 1.0, the outcome is exactly as expected. If it is less than 1.0, actual mortality is lower than expected.
At the time of the survey, the average RAMR for all surgeons taken together was 0.22. The most extreme risk avoider in my survey is surgeon 3, whose RAMR is 0.1, a very good result. Next come surgeons 12 and 13: their RAMRs are 0.4 and zero, respectively. Looking at the most risk-taking surgeons, they are surgeons 4, 6, and 11. Their RAMRs are 0.25, 0.6, and 0.25. The surgeons in the middle of the scale also have wide variation in their RAMRs, ranging from zero to 0.33. There seems to be no correlation whatsoever between risk propensity and successful outcomes. We have risk-takers and risk-avoiders achieving broadly similar results, with similar variations within each group. So there is no association between individual surgeons’ risk propensity and the outcomes for their patients. The average results at Papworth are excellent overall. Some of our surgeons perform slightly better or slightly worse than our average, but this appears to be totally unrelated to their individual risk propensity: a personality trait that shows a propensity to take risk or a propensity to avoid it does not appear to directly influence outcomes.
One might be a little disappointed by this seemingly negative finding. After all, there is good evidence that it is obsessive risk avoidance that has made aviation so safe, and that one of the problems in medicine is the comparative dearth of such risk avoidance. One would naturally expect that excessively risk-taking surgeons should encounter more problems, and therefore have worse results. The facts suggest otherwise. Does that mean that risk propensity has no bearing at all on surgical outcomes?
It would be perfectly reasonable to postulate that a surgeon’s propensity to take risk is not immutably etched in concrete. Regardless of his or her position on the risk propensity scale, our surgeon is likely to vary in risk-taking within a certain range. I, for one, know that I am sometimes more gung-ho than at other times. Sometimes, I am adventurous. At other times, I am hyper-careful. The variation in my own risk propensity seems to be directly related to the life events I am experiencing at the time.
Does this variation in risk propensity apply to everyone? Common sense would suggest that it probably does. A driver who has just been caught for speeding is more likely to observe the speed limit. A smoker whose best friend has just died of lung cancer is more likely to consider ditching the cigarettes. An investor who has just lost a tidy sum on the stock market is unlikely to feel bullish when the next risky investment opportunity presents itself. We are human, and our behaviour is strongly modified by our reaction to certain events. I am not making this up: cognitive psychologists have studied these phenomena for years, and there is even a branch of science called ‘heuristics’, which focuses on the link between experience, learning, and behaviour. Furthermore, evolutionary psychologists believe that this type of judgement bias is actually hardwired in our genes, and can be explained in Darwinian evolutionary terms. There are some truly peculiar traits in our behaviour towards risky situations. Some of these traits do not even make sense, but we guess that they are there because they must have been in some way beneficial for survival in the ancestral environment 100,000 years ago or thereabouts. As a result, certain types of events in your life make you more risk-averse than usual, and happenings of another kind make you more gung-ho than usual.
Let us now come back to surgeons. We know that, like every other group of professionals, surgeons come in all shapes and sizes: male and female, short and tall, fat and thin, black and white. We have also established that they differ in personality, and, in particular, we now know for certain that they differ in their position on the risk propensity scale. I also know that there is not a shred of evidence that sex, size, and colour have any impact at all on surgeon performance, though it seems reasonable that a surgeon’s age, experience, manual dexterity, conscientiousness, knowledge, and sound judgement may influence surgical outcomes. These factors are likely to have an impact, but what about the small variation in an individual surgeon’s risk propensity? Does that affect the results of surgery?
Recall those two studies that had somewhat surprising findings. In the study of deaths on the operating table, we found that such a death did in fact have an adverse impact on surgical performance in the subsequent 48 hours, so that patients operated on in the immediate aftermath of an on-table death fared worse than others. This in itself was predictable, but what was surprising was that the effect appeared to be worse if the preceding death on the table was a high-risk or emergency (somewhat ‘expected’) death. In fact, this finding was diametrically opposite to the views of the clinicians who were surveyed about the subject, and who overwhelmingly believed with conviction that an unexpected death in a low-risk patient is precisely the sort of thing that can affect subsequent performance.
The second study looked at holidays and surgical performance. Here there were no expectations. One could envisage that a break would do the surgeon some good, and deliver him or her refreshed and raring to go, but one could equally well imagine that a long time away from the operating table would deliver an uncertain surgeon who has been somewhat de-skilled and who will perform a little worse until back in the groove. The findings showed that there was no loss of skill after a holiday, and that results were, if anything, better than usual on the first day back. This in itself was not too surprising, but the outcomes of patients operated the day before a surgeon went on holiday were surprisingly bad: the mortality of these patients was more than double the mortality of those operated on the first day back from holiday.
We do not know the true cause behind these unexpected findings, but here is a possible explanation for both.
We have established that surgeons vary in their risk propensity. We know that this variation between surgeons does not by itself relate to variation in outcomes. We also know that the risk propensity of an individual surgeon is not fixed, but varies in a way that is affected by the events of life, so that each surgeon’s risk propensity spans a range. So perhaps surgeons are safest and best when they are operating towards the risk-averse end of their own personal risk-propensity range.
Consider a hypothetical surgeon in action within the context of the two previous studies. He is an ordinary bloke, with a risk-propensity range somewhere in the middle. He is neither an excessive risk-taker nor so obsessively risk-averse that he is paralysed with fear in the operating room. Most days, he works in the middle of his risk-propensity range, then something happens that moves him to one or the other end of his range. Let us look at some scenarios.
Scenario 1
Our surgeon is on call for emergencies, and is presented with an almost impossibly high-risk patient. The situation is one of kill-or-cure salvage: he either operates immediately or the patient will die. It is 10.00 p.m. Many people feel the case is hopeless. In fact, he knows of many colleagues who would not dream of offering surgery to a patient of such high risk, but he also knows that, without his intervention, the patient will be dead soon. He decides to go ahead. There are both expected difficulties and unexpected complications. The operation takes 12 hours instead of four hours, as would normally be the case. He is up all night. His routine, next day’s operating list of two straightforward CABG operations is going to be delayed. At 10.00 a.m. the following day, he has tried everything to save the emergency patient, and all his efforts have failed. He has been ‘firefighting’ all night, and finally decides to give up, as the situation is hopeless. The patient dies on the operating table. He sends for the next routine CABG case, and is ready to start operating at about 11.00 a.m. He is very tired, and slightly fed up with the way the night has turned out, but wants to get on and finish these ‘easy’ cases as quickly as possible so he can get home and rest before the dinner party he has promised to attend. As he now embarks on the routine case, with a ‘bring ’em on’ attitude, is he likely to be more risk averse or risk-taking? I believe he is undoubtedly going to be taking more risks than usual.
Scenario 2
Our same surgeon has been having a good run, with great results. He is happily operating on a low-risk young patient in a relaxed and pleasant environment. The operation is easy, and he is enjoying operating-room banter with the staff when something suddenly and unexpectedly goes catastrophically wrong. The otherwise fit and healthy patient, who had rightfully expected a normal life span after correction of a heart defect, dies on the operating table: an unmitigated and totally unexpected disaster. The next case is a standard CABG operation. When he approaches it, is he likely to be more risk averse or risk-taking? Of course he is going to be risk-averse. In fact, he will be terrified: he has had the rug pulled out from under him, and has been left questioning and doubting everything, including himself and his purpose in life. He will certainly not be taking risks.
Scenario 3
Our surgeon is operating on the last day before a much-needed two-week holiday in the sun. He and his family have a flight to catch at 7.00 p.m. that very evening, but he thinks he will finish operating in time to get home and help with the packing. There are other important last-minute arrangements to be made, such as to book parking at the airport and fill the car with fuel. Sadly, that is not all: there is a large pile of patient correspondence to be dealt with before he goes, and he knows that, if it is not dealt with now, it will have grown to unmanageable proportions on his return. Added to all of that are a couple of administrative meetings that simply must be squeezed in over lunch before his leave begins. He has not had a break from work for three months, and this holiday is so important to him. His mind is already on the flight and the beach, but these cases must be operated first. Is he likely to be taking risks? Definitely.
Scenario 4
Our surgeon has just spent two weeks on a fantastic holiday in the sun. Surgery, administrative work, and hospital-management problems seem miles away. He is re-acquainted with his loved ones, with himself, and with what he really enjoys doing when he is not operating. He is back, refreshed, and happy but has not set foot in an operating room for more than two weeks. It’s Monday morning, the first patient is on the table, and our surgeon hopes that he is still up to the task (especially since he found out on his return that the patient he operated on the day before his leave did not do so well). Does he take risks? Obviously not: he will be at his most risk-averse.
In scenario 2, a young patient died unexpectedly on the operating table, and, in scenario 4, the surgeon is a tad uncertain about his ability to operate after a long absence. Both contribute to a mindset that is hyper-aware of what can go wrong, and both engender extra care to avoid errors. This is analogous to the situation with Jehovah’s Witnesses. By categorically refusing blood transfusion, they take away from the surgeon the possibility of giving blood in case of bleeding, and thus withdraw one of the surgeon’s safety nets. A surgeon faced with a Jehovah’s Witness on the operating table is naturally going to be more risk-averse when it comes to bleeding, and will take a lot of care to ensure that the risk of bleeding is minimised. It is no surprise that such patients bleed so little.
If my hunch is true, and surgeons are safest and best when they are operating towards the risk-averse end of their own personal risk-propensity range, it goes a long way to explain both the surprising findings in the holiday study and the study of deaths on the table. It also means that we have at our disposal a new method to improve surgical outcomes, over and above good training, good technique, conscientiousness, and a high standard of care, over and above robust quality monitoring and even the Hawthorne effect: we should be able to improve surgical outcomes by modifying the risk-taking behaviour of surgeons. How do we do this? I am not exactly sure yet, but I have a few ideas. For a start, we need a hard measure of risk propensity, one that is more robust than surgeons’ own perception of themselves and others, and we also need a host of interventions specifically designed to modify such propensity. Preliminary exploratory work suggests that all of this may indeed be possible, but it is beyond the scope of this book, and will form the basis of some future research on this fascinating topic. In other words, watch this space.