11.1 Introduction
Microbes dominated the earth for at least 2.5 billion years before multicellular life appeared in the biosphere (Hooper and Gordon 2001; Ley et al. 2008). In fact, animals have been living and evolving in a microbial world (Margaret et al. 2013). Microbes inevitably colonized animals and coevolved through the process of interactions with their hosts (Clayton et al. 2018). Microorganisms can inhabit multiple parts of a host’s body, such as the skin, oral cavity, sex organs, and the gastrointestinal (GI) tract. They are usually recognized as the microbiome of a particular host. As an essential part of the host’s body, the microbiome plays an important role in host physiology by influencing nutritional intake, metabolic activity, and immune homeostasis (Turnbaugh et al. 2006; Greenblum et al. 2012; Hooper et al. 2012). Thus, the complex relationship between hosts and their microbiomes provides a unique opportunity for understanding mammalian adaptation and evolution (Hird 2017).
As the most complex and diverse ecological system of the mammalian body, the GI tract is colonized by bacteria, archaea, fungi, and viruses (Underhill and Iliev 2014). Previous studies have revealed that the gut microbiota play an important role in immune regulation, vitamin synthesis, energy acquisition, and disease risk reduction of the host (Turnbaugh et al. 2006; Hooper et al. 2012; Sharon et al. 2012). For example, all vertebrates lack cellulase, the enzymes, which help them to digest the fiber in their food, and are reliant upon intestinal microbes for its production (Yokoe and Yasumasu 1964; Mackie 2002). As a result, mammals maintain rich gut microbial communities, which are beneficial for the digestion of cellulose and hemicellulose abundant in the foods they ingest (Amato et al. 2015).
Nonhuman primates (NHPs) evolved in tropical forest habitats and radially spread to woodland, savanna and montane environments (Hanya et al. 2011). In order to adapt to the resultant temporal food resource shifts and to maintain ordinary functions of the body, NHPs have evolved many anatomical and behavioral strategies (Rodman and Cant 1984). For example, some primates have evolved specialized stomachs and teeth, while many others have evolved distinctive habitat use patterns, ranging behavior and social organization (Milton and May 1976; Chivers et al. 1984; Cachel 1989; Yamada and Muroyama 2010; Hanya and Chapman 2013). Generally, NHPs prefer to eat high-quality food rich in lipids, proteins, and carbohydrate, such as fruits and young leaves. However, these foods are not always available year-round (Rodman and Cant 1984; Ungar 1995; Hanya et al. 2011; Hanya and Chapman 2013). NHPs living in temperate environments can only eat mature leaves, gum and roots during winter (Fan et al. 2009). These low-quality foods usually contain high proportions of cellulose and hemicellulose, which are difficult to digest (Coley 1983; Burrows 2010). In response to ecological pressures, many primates also use behavioral strategies such as adjusting their activity budgets and foraging patterns to help overcome periods of food scarcity (Zhou et al. 2007; Starr et al. 2012; Hanya and Chapman 2013; Campera et al. 2014). However, how wild primates digest low-quality foods high in cellulose during the winter has not been well understood (Amato et al. 2014).
Although low-quality foods, including woody plants, mature leaves, fungi, mature leaves, and plant exudates are difficult to digest, more recent research related to the above has revealed that these foods can be broken down and utilized by the intestinal microbes of NHPs. For this reason, the gut microbiota may provide a way to understand how primates adapt to ecological pressures during periods of food scarcity. Since the gut microbiome is dominated by bacteria, many studies on the feeding ecology of mammals have focused solely on the bacterial microbiome (Qin et al. 2010). Other common elements, such as gut fungi, also appear to affect the health and nutrition of animals, but this too is not yet well understood (Huffnagle and Noverr 2013; Underhill and Iliev 2014; Sokol et al. 2017). Although intestinal fungi have a much smaller number of cells compared to bacteria, the evidence suggests that gut mycobiota can also play an important role in host health by affecting gut bacterial composition (Hoffmann et al. 2013), interacting with immune cells, and assisting in the metabolic activity of the host (Hajishengallis et al. 2011; Romani 2011; Iliev and Underhill 2012; Rizzetto et al. 2014). Therefore, it is important to integrate information about the role of gut bacteria and fungi when discussing the role of the gut microbiome in the evolution of animal dietary strategies.
NHPs and humans have extensive similarities in their genetic characteristics, physiology and morphology. Very important animal model systems, NHPs are extremely important for understanding human physiology, behavior, cognition, health and evolution (McCord et al. 2014; Ren et al. 2015). Therefore, compared with other laboratory animals, NHPs have an advantage in helping us to understanding the role of the gut microbiome in human health maintenance, as well as the evolutionary relationships involving the gut microbiome, host diet and evolution.

A female Tibetan macaque carrying a baby is foraging for food in winter (Photo credit: Qixin Zhang)
In this chapter, we will summarize our research on the composition of the bacterial/fungal community and microbial diversity of Tibetan macaques and analysis of the factors influencing variation in the microbiome across age, sex and season in this species. Furthermore, we use this information to discuss the role of the Tibetan macaques’ gut microbiome in relation to the evolution of their feeding ecology.
11.2 Gut Microbiome of Tibetan Macaque
11.2.1 Composition of Gut Bacteria
The dominant phyla of gut bacteria detected in several non-human primate species
Monkey’s name | Dominant phyla | Captive/wild | Study | ||
|---|---|---|---|---|---|
Firmicutes | Bacteroidetes | Proteobacteria | |||
Lemur Catta | + | + | Captive | McKenney et al. (2015) | |
Varecia variegata | + | + | Captive | McKenney et al. (2015) | |
Propithecus coquereli | + | + | Captive | McKenney et al. (2015) | |
Rhinopithecus bieti | + | + | + | Wild | Xu et al. (2015) |
Nycticebus pygmaeus | + | Wild | Xu et al. (2013) | ||
Gorilla beringei | + | + | Wild | Ochman et al. (2010) | |
Gorilla gorilla | + | + | Wild | Ochman et al. (2010) | |
Pan troglodytes | + | + | Wild | Ochman et al. (2010) | |
Macaca thibetana | + | + | Wild | Sun et al. (2016) | |
11.2.2 Composition of Gut Fungi
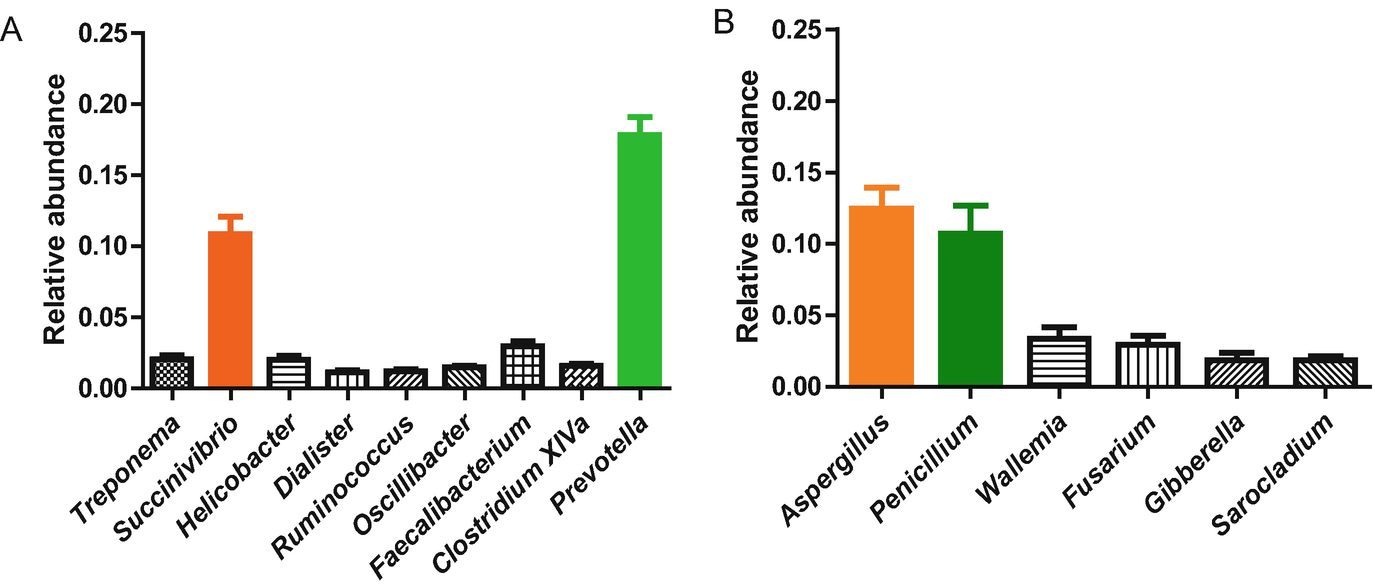
Previous studies on humans and mice have revealed that the mammalian gut is rich in fungi, dominated by three phyla: Ascomycota, Basidiomycota, and Zygomycota (Iliev and Underhill 2012; Qiu et al. 2015; Mar et al. 2016; Strati et al. 2016; Wheeler et al. 2016; Sokol et al. 2017). However, little is known about the gut fungi in NHPs. Using the Illlumina Miseq platform and primers of the ITS region (Bokulich and Mills 2013; Sun et al. 2018) first reported that the gut mycobiota of Tibetan macaques was dominated by two phyla Ascomycota and Basidiomycota (Sun et al. 2018), this is consistent with previous studies on mammalian gut mycobiomes. In addition, other phyla such as Zygomycota, Chytridiomycota, Glomeromycota and Rozellomycota were also detected in this macaque species. At the family level, Trichocomaceae shows the highest relative abundance (24.70%). At the genus level, the gut mycobiota of Tibetan macaques are rich in Aspergillus and Penicillium (relative abundance more than 10% respectively). Furthermore, by defining the core families and genera (both present in >90% of the samples with an average relative abundance > 0.01), Sun et al. (2018) revealed that six core taxa, namely Trichocomaceae, Nectriaceae, Davidiellaceae, Aspergillus, Penicillium and Fusarium can be detected in the gut of Tibetan macaques. The relative abundances of fungal genera (>1%) are presented in Fig. 11.2b. The presence of core taxa in the gut of this macaque suggests that the fungi in their guts are not random and exist as a relatively stable fungal community.
11.3 Factors Affecting the Gut Microbiome in Tibetan Macaques
11.3.1 Affects of Age, Sex, and Season on Gut Bacterial Microbiome
It is well known that many internal and external factors can affect the diversity and composition of an animal’s gut microbiota, such as age, sex and seasonal change. Based on data from the Illlumina Miseq platform and linear mixed models, Sun et al. (2016) evaluated factors affecting gut microbial diversity in Tibetan macaques. The results showed that sex and age had no significant effect on the alpha diversity (including Shannon index, Chao 1, OTU richness and ACE) of the gut bacterial microbiome, as well as the beta diversity (evaluating by unweighted and weighted UniFrac distances) (Sun et al. 2016). These results differ from previous studies in humans and other vertebrates (Yatsunenko et al. 2012; Bolnick et al. 2014), but are consistent with those in chimpanzees and baboons (Degnan et al. 2012; Tung et al. 2015).
Seasonal factors on the other hand have a strong effect on the composition and diversity of the gut bacterial microbiome in Tibetan macaques (Sun et al. 2016). For example, the Shannon diversity index of the gut bacterial microbiome was significantly different between winter and spring. In addition, a significant seasonal separation of beta diversity evaluating by weighted UniFrac distances and unweighted UniFrac distances (Permanova tests, p < 0.05 and 0.01 respectably) were detected. Furthermore, three phyla and 20 known genera showed significant differences between the two seasons. The representative taxa rich in winter samples were Proteobacteria, Spirochaetia, Succinivibrio, Clostridium sensu stricto and Treponema, but Firmicutes and Prevotella were richest in spring samples. Differences in the composition of gut bacteria also resulted in the variations of predicted metagenomes between winter and spring. Using PICRUSt and LEfSe tests, Sun et al. (2016) found that seven KEGG pathways of Tibetan macaques’ gut bacterial microbiome, including Glycan Biosynthesis and Metabolism, Amino Acid Metabolism, Signal Transduction, Cell motility, Transport and Catabolism, Neurodegenerative Diseases and Endocrine System, were significantly enriched in the winter. However six other metabolic pathways Energy Metabolism, Carbohydrate Metabolism, Cellular Processes Transcription, Signaling and Metabolism of Cofactors and Vitamins and Enzyme Families were significantly enriched in the spring (Sun et al. 2016). The potential functions of the gut microbiome in different seasons of our study group will be discussed in Sect. 11.4.
11.3.2 Gut Fungal Microbiome Affected by Age, Sex, and Season
Although previous studies in humans have revealed that age and sex can influence the composition and diversity of the gut fungal microbiome (Strati et al. 2016), little information is available for wild living primates. Using LEfSe analysis, Sun et al. (2018) first reported that only one fungal genus Sarocladium was enriched in the old age group. By comparing males and females, the family Mycosphaerellaceae and genus Devriesia were particularly enriched in females, while the phylum Ascomycota and family Tetraplosphaeriaceae were enriched in males (Sun et al. 2018). These results indicated that the effect of sex and age on the composition of gut fungi was not as strong as that found in humans. In addition, we found evidence of marked seasonal variation in the composition of this macaque species’ gut mycobiota. Fifteen taxa had significant variation across the four seasons. The abundant taxa in each season were as follows, Autumn: Wallemiaceae, Hypocreaceae, Wallemia, Trichoderma; Spring: Sclerotiniaceae, Nectriaceae, Ciboria, Fusarium, Gibberella, Sarocladium and Talaromyces; Summer: Trichocomaceae and Penicillium; Winter: Devriesia and Teratosphaeriaceae. This result is the first report to find strong seasonal influences on primate gut mycobiota, indicating the potential relationship between gut mycobiota and seasonal changes in host diet.
Different from previous studies in humans, no evidence indicates that age or sex strongly affect the alpha diversity of Tibetan macaque gut mycobiota (Sun et al. 2018). This result is likely because even individuals living in the same environment have differences in their dietary preferences. In addition, using PCoA and PERMANOVA tests, we found that there existed significant variation in beta diversity among different age and sex classes. Interestingly, seasonal change can significantly affect alpha and beta diversity, as well as the composition of the gut fungal microbiome.
11.4 Functions of the Gut Microbiome in Tibetan Macaque Feeding Ecology
Feeding ecology plays an important role in understanding the evolution of NHPs (Lambert 2011). With advances in sequencing technology, many studies have revealed that the gut microbiome aids in the digestion of some food resources, especially those rich in cellulose and hemicellulose, substances which are difficult to digest. Therefore, this important adaptive mechanism has attracted increasing attention in recent years. Tibetan macaques living in a highly seasonal ecosystem with strongly seasonal changes in rainfall, temperature, food resources, and home range shifts (Xiong and Wang 1988; Zhao 1999). Previous studies have suggested that these environmental factors are closely reflected in the composition of the gut microbiome (Amato et al. 2015; Chevalier et al. 2015; Maurice et al. 2015; Wu et al. 2017). Therefore, our study subjects may provide an important model for understanding the functions of the gut microbiome in primate feeding ecology, especially for primates living in highly seasonal ecosystems.
11.4.1 Gut Bacterial Microbiome and the Feeding Ecology of Tibetan Macaques
It is well known that the gut bacterial microbiome can help hosts to digest food resources more efficiently, and they also can change according to variation in host diet across time and space, helping the host to meet its nutrient and energy requirements (Hooper et al. 2002; Donohoe et al. 2011; Koren et al. 2012; Amato et al. 2014; Chevalier et al. 2015). As a species living in a highly seasonal ecosystem, Tibetan macaques depend mostly on a plant diet for their subsistence, including leaves, grass, roots, fruits, and flowers, and they usually shift home ranges to adapt to seasonal changes in food availability (Xiong and Wang 1988; Zhao 1999). The diverse and responsive gut bacterial community of wild-living Tibetan macaques can be considered an important adaptation for their foraging lifestyle. The presence of the relatively abundant fiber-degrading bacteria of Firmicutes and Bacteroidetes in their gut can help them to utilize the heavily plant-based diet. Some well-known fiber-degrading bacteria genera detected in the gut of Tibetan macaques, such as Succinivibrio and Clostridium, suggest that the gut bacterial microbiome plays an important role in the Tibetan macaques’ feeding ecology.

Food type change and adaptive shifts of the gut bacterial microbiome in Tibetan macaques. The predicted metagenomes of KEGG pathway and bacterial taxa of the Tibetan macaque gut microbiome, which significantly increased in winter, are beneficial for the digestion of cellulose and hemicellulose rich foods in winter, and the other bacteria significantly increased in spring, which are beneficial for the digestion of pectin, carbohydrate and simple sugar rich foods which are abundant in spring
During winter, the representative gut bacterial taxa Proteobacteria, Succinivibrio and Clostridium significantly increased, and the predicted metagenomes of glycan biosynthesis and metabolic pathways significantly increased in winter samples (Sun et al. 2016). The genus Succinivibrio, which usually is detected in rumen microbial ecosystems, ferment glucose efficiently by producing acetic acid and succinic acid (de Menezes et al. 2011), as well as being beneficial to the metabolism of different types of fatty acids (Van Dyke and McCarthy 2002). Another genus Clostridium contains many organisms which produce cellulose and hemicellulose-digestive enzymes (Van Dyke and McCarthy 2002; Zhu et al. 2011). The significant increase of the genus Clostridium in winter samples is very beneficial for the digestion of cellulose and dietary fiber. In addition, evidence from black howler monkeys, humans and mice reveals that Proteobacteria are highly correlated with energy acquisition (Bryant and Small 1956; Koren et al. 2012; Amato et al. 2014; Chevalier et al. 2015). In response to cold weather, primates experience an increase in energy loss (Tsuji et al. 2013), and Tibetan macaques are no exception. The increase of Proteobacteria is beneficial for coping with cold weather. In conclusion, the gut bacterial microbiome in winter increases the efficiency of dietary fiber digestion in Tibetan macaques, which helps them to meet their energy requirements.
In addition, Sun et al. (2016) found that Firmicutes and Prevotella were significantly enriched in the Tibetan macaque gut during spring. During this time, the diet consists mainly of young leaves, flowers and bamboo shoots, foods that are richer in digestible pectin, carbohydrates and simple sugars, compared to mature leaves (Xiong and Wang 1988; Zhao 1999; You et al. 2013). The genus Prevotella is associated with the digestion of carbohydrates simple sugars, pectin and hemicellulose (Amato et al. 2014). The significant increase of genes related to carbohydrate metabolism and energy metabolism pathways in spring samples also indicates that the gut bacterial community helps break down and make accessible these high-quality food resources, beneficial for macaques to recover from energy loss experienced during the cold conditions of winter.
11.4.2 Gut Fungal Microbiome and Feeding Ecology of Tibetan Macaques
Compared with the gut bacterial microbiome, there is very little data on the primate gut fungal microbiome, and the role of gut fungi in primate foraging ecology is unknown. Sun et al. (2018) first reported the diversity and composition of Tibetan macaque gut mycobiota. Three genera Aspergillus, Penicillium and Fusarium were detected as normal inhabitants in the gut. The two most dominant genera, were Aspergillus (12.46%) and Penicillium (10.72%). It has been reported that the anaerobic species of these two genera can produce cellulolytic and hemicellulolytic enzymes, which are beneficial to cellulosic biomass degradation (Boots et al. 2013; Liao et al. 2014; Shuji et al. 2014; Solomon et al. 2016; Trinci et al. 1994). Similarly, as robust cellulose and hemicellulose degrader, the genus Fusarium was reported in a recent study (Huang et al. 2015). At certain times of the year, Tibetan macaques depend on a plant diet, high in cellulose and hemicellulose (Xiong and Wang 1988; You et al. 2013; Campbell et al. 2001). Our previous studies indicated that the dominant genera of Tibetan macaque gut mycrobiota may play an important role in the digestion of these and other plant items.
In addition, the gut fungal microbiome of Tibetan macaques changed with the seasonal change in dietary food content. Fifteen taxa were detected to be significantly enriched in one of the four seasons (Sun et al. 2018). This result indicates that the gut mycrobiota can response to dietary shifts. It has been proposed that the gut mycobiota response to seasonal dietary shift is beneficial to the hosts’ nutritional and reproductive needs (Noma et al. 1998; Tsuji et al. 2013). However, knowledge about the functions of the particular fungi in the mammalian gut is limited (Milton and May 1976), as are genomic databases and metabolic maps of gut fungi (Huffnagle and Noverr 2013), making it difficult to explain the relationship between mycobiota composition and primate feeding ecology. Based on the available information about the genus Penicillium (Shuji et al. 2014), we hypothesize that this summer season enriched genus may aid in the digestion of mature leaves, which contain a higher proportion of cellulose and hemicellulose. However, a possible explanation as to why this genus was not enriched during winter could be that the ingestion of fallen nuts in winter caused the amount of Penicillium to decrease due to some chemical interaction (Maria et al. 2014). Secondly, it has been reported that high diversity of gut mycobiota can improve the utilization efficiency of plant fiber consumption (Bauchop 1981; Akin et al. 1983; Denman and Mcsweeney 2010). Thus, the gut mycobiota with high alpha diversity during the winter, reported in our previous study, may be beneficial for Tibetan macaques to digest dietary fiber. In conclusion, the gut fungal mycobiota detected in Tibetan macaque provides evidence for the role of gut fungi in primate foraging ecology. More attention needs to be paid to this in future studies.
11.5 Conclusions and Future Directions
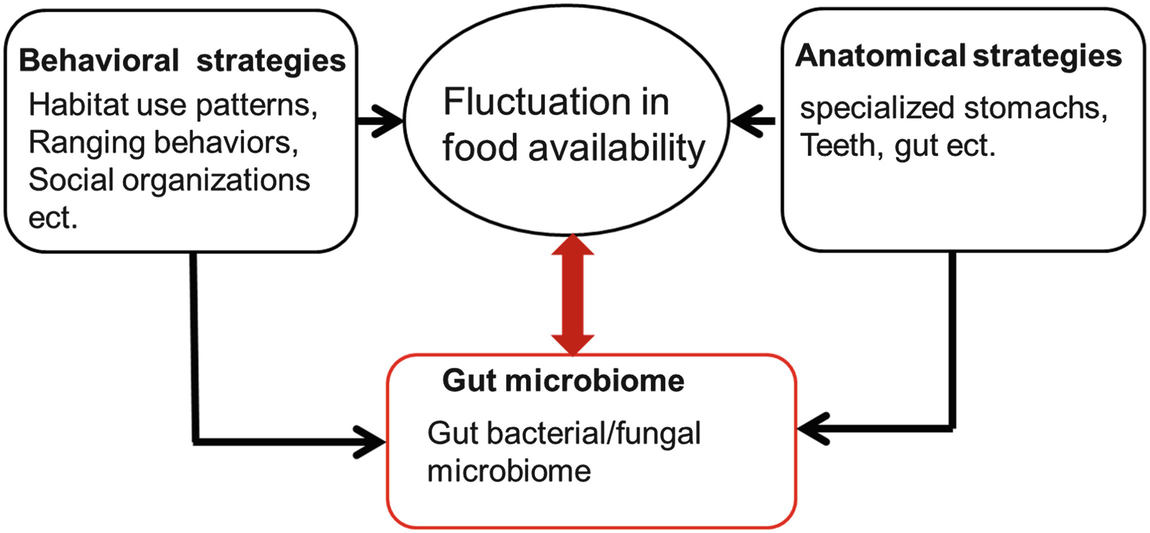
Adaptive mechanisms of wild primates in response to fluctuations in food availability and food quality
Many thanks to the Huangshan Garden Forest Bureau for their permission and support of this work. We also gratefully acknowledge Paul A. Garber, Lori K. Sheeran and all members of our research group for their excellent work on the gut microbiome of Tibetan macaques. This chapter was supported by grants from the National Natural Science Foundation of China (No. 31870371, 31400330, 31172106), the Special Foundation for Excellent Young Talents in University of Anhui Province, China (No. 2012SQRL018ZD) and the Initial Funding for Doctoral Research in Anhui University (No. J01003229).

Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.