Measurement
Scientists don’t use British measurement units such as ounces, miles, and gallons; instead, they use the metric system. The metric system has been used throughout most of this chapter, but it’s time for a more detailed examination. The key idea of the metric system is to designate one base unit for every kind of measurement, and then make bigger or smaller units by adding prefixes to it (groups of letters added to the beginnings of words, like anti– or pro–). For example, the base unit for length in metric is called the meter (m), which is just over a yard (about 39.4 inches).
To measure small things like firearm ammunition or large things like the distance between cities, a prefix is added. Milli– means
 , so a millimeter (mm) is
, so a millimeter (mm) is
 the size of a meter. A 9 mm handgun like the Beretta M9 (the US military’s standard sidearm) fires rounds that are .009 m in bullet diameter. A centimeter (cm) is 10 times as big as a millimeter, and there are about 2.54 cm in one inch. A kilometer (km) is 1,000 meters. Metric measurements typically have two- or three-letter symbols (like mm, cm, and km), which are usually the first letter of the prefix followed by the first letter of the base unit.
the size of a meter. A 9 mm handgun like the Beretta M9 (the US military’s standard sidearm) fires rounds that are .009 m in bullet diameter. A centimeter (cm) is 10 times as big as a millimeter, and there are about 2.54 cm in one inch. A kilometer (km) is 1,000 meters. Metric measurements typically have two- or three-letter symbols (like mm, cm, and km), which are usually the first letter of the prefix followed by the first letter of the base unit.
| Prefix | Symbol | Value relative to base unit |
|---|---|---|
| mega | M | 106 or 1,000,000 |
| kilo | k | 103 or 1,000 |
| hecto | h | 102 or 100 |
| deka | da | 101 or 10 |
| base (no prefix) | — | 1 |
| deci | d | 10-1 or
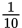 or 0.1
or 0.1 |
| centi | c | 10-2 or
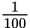 or 0.01
or 0.01 |
| milli | m | 10-3 or
 or 0.001
or 0.001 |
| micro | µ (the Greek letter mu, to avoid confusion with “mega”) | 10-6 or
 or 0.000001
or 0.000001 |
The most common prefixes (and the most likely to come up on the ASVAB) are milli–, centi–, kilo–, and sometimes mega–. The other prefixes on the table are included for the sake of completion, but it’s not important to memorize them, nor other prefixes beyond those listed here. Know both the prefix names as well as their symbols, as the symbol (for example, km for kilometer) is more likely to come up in a calculation question than the name.
Applying the prefix scheme to other measurements, mass is measured in a base unit of grams (g). Therefore, large objects can be measured in kilograms (kg), and very small things can be measured in milligrams (mg); for example, the amount of certain minerals and nutrients per serving as listed on a food package’s nutritional information tend to be in milligrams. There are approximately 28.3 grams in an ounce, and a mass of one kilogram will have a weight of approximately 2.2 pounds (at the surface of the Earth).
Volume is the measurement of three-dimensional space. A cube (square box) which is one centimeter on a side can be called just that: a cubic centimeter (cc); but it can also be called a milliliter (mL). The definition of the milliliter automatically implies the liter (L): the prefix means a milliliter is one-thousandth of a liter, which is the same as saying a liter is equal to one thousand milliliters. A liter is equal to slightly more than a quart in liquid measure, or about 33.8 ounces, with about 3.79 liters in a gallon.
Time is measured in seconds (s) and metric prefixes are used when smaller units are required (as in milliseconds). However, minutes (min) and hours (h) are also common.
Finally, the metric system equivalent of temperature is the Celsius scale, also known as degrees centigrade. According to the Fahrenheit scale, which is more familiar to most people in the United States, water freezes at 32°F and boils at 212°F. On the Celsius scale, water freezes at 0°C and boils at 100°C. The general equations for converting from the Fahrenheit scale to the Celsius scale, or vice versa, are as follows:


Finally, there is one other temperature scale commonly used by scientists, known as the Kelvin scale, or the absolute zero scale. Absolute zero is the temperature at which matter has no heat and its molecules are completely still; in theory, absolute zero is the lowest temperature possible. On the Kelvin scale, absolute zero is set at 0 K, which is equal to −273°C. Otherwise, the Kelvin scale uses the same increments as degrees Celsius, so that water freezes at 273 K and boils at 373 K. Note that there is no degree symbol when writing out temperatures in the Kelvin scale.
The unit types discussed here are not exhaustive. Different base units come up in different areas of science, but the beauty of metric standards is that the value of each prefix is a constant. Metric prefixes will be applied in new contexts elsewhere in this chapter, and again in chapter 10: Electronics Information.
| Question | Analysis |
|---|---|
| In a chemistry lab, the experimental procedure says to add 50 mL of room temperature water to a beaker. How many liters are in 50 mL? | Step 1: The question asks for the value of 50 mL converted to L units. |
| Step 2: The prefix milli– has a value of 0.001 of the base unit. Since L are 1,000 times larger than mL, the equivalent number in L will be 1,000 times smaller than the number in mL. The decimal will move by three steps. 50 mL =
0.05 L is equivalent to 50 mL. |
|
| Step 3: The solution is 0.05 L. | |
| (A) 50,000 L (B) 5 L (C) 0.5 L (D) 0.05 L |
Step 4: Answer choice (D) is correct. |
Now you try one:
-
In an experiment, you must measure out 0.13 kg of sodium chloride. However, your instruments are only set to work with gram units. Convert 0.13 kg into grams. - 1.3 g
- 13 g
- 100 g
- 130 g
Explanation
Choice (D) is correct. Since there are 1,000 g in 1 kg, and this is a conversion to the smaller unit value, simply move the decimal place three places to the right (once for every 0 in 1,000).
 L
L