Chemistry
Elements and the Periodic Table
Chemistry is the science dealing with the composition and properties of matter, and with the reactions by which matter is produced from or converted into other types of matter. Matter is anything that has mass and occupies space, and any form of matter has certain chemical properties based upon its molecular composition. To start, here are some basic terms:
Element: A pure type of matter that cannot be separated into different types of matter by ordinary chemical methods. All matter is composed of elements, and all the known elements are listed on the Periodic Table of Elements (see the figure on the next page).
Atom: The smallest component of an element that still retains the properties of the element. An atom may combine with similar particles of other elements to produce compounds. Atoms consist of a complex arrangement of electrons in motion about a positively charged nucleus containing protons and (except for hydrogen) neutrons.
Proton: A subatomic particle found in the atom’s nucleus that carries a positive electric charge.
Neutron: A subatomic particle found in the atom’s nucleus that does not have an electric charge and is therefore neutral.
Electron: A subatomic particle that orbits the nucleus of an atom. An electron carries a negative charge and has a miniscule mass compared to the other sub-atomic particles (both neutrons and protons are more than 1,800 times as massive as an electron). Ordinarily, an atom has the same number of negative electrons around the nucleus as the number of positive protons in the nucleus.
Structure of an Atom
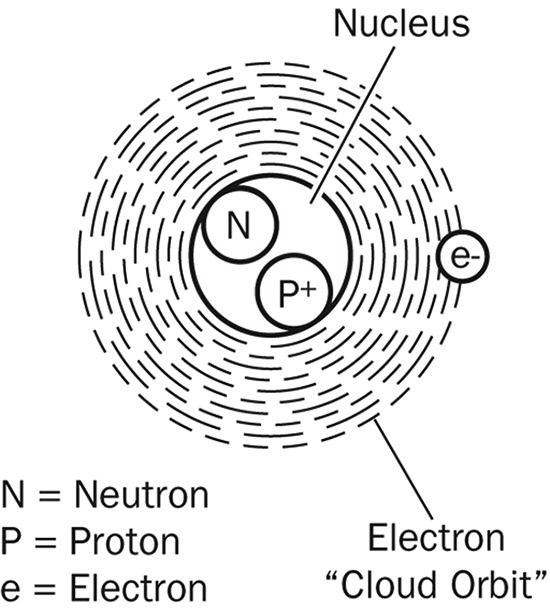
Molecule: The smallest multi-atom particle of an element or compound that can exist in the free state and still retain the characteristics of the element or compound. The molecules of elements consist of two or more similar atoms; the molecules of compounds consist of two or more different atoms.
Through decades of research, scientists have organized all the known elements into a structure called the Periodic Table, which conveys multiple pieces of information about each element.
Periodic Table of the Elements
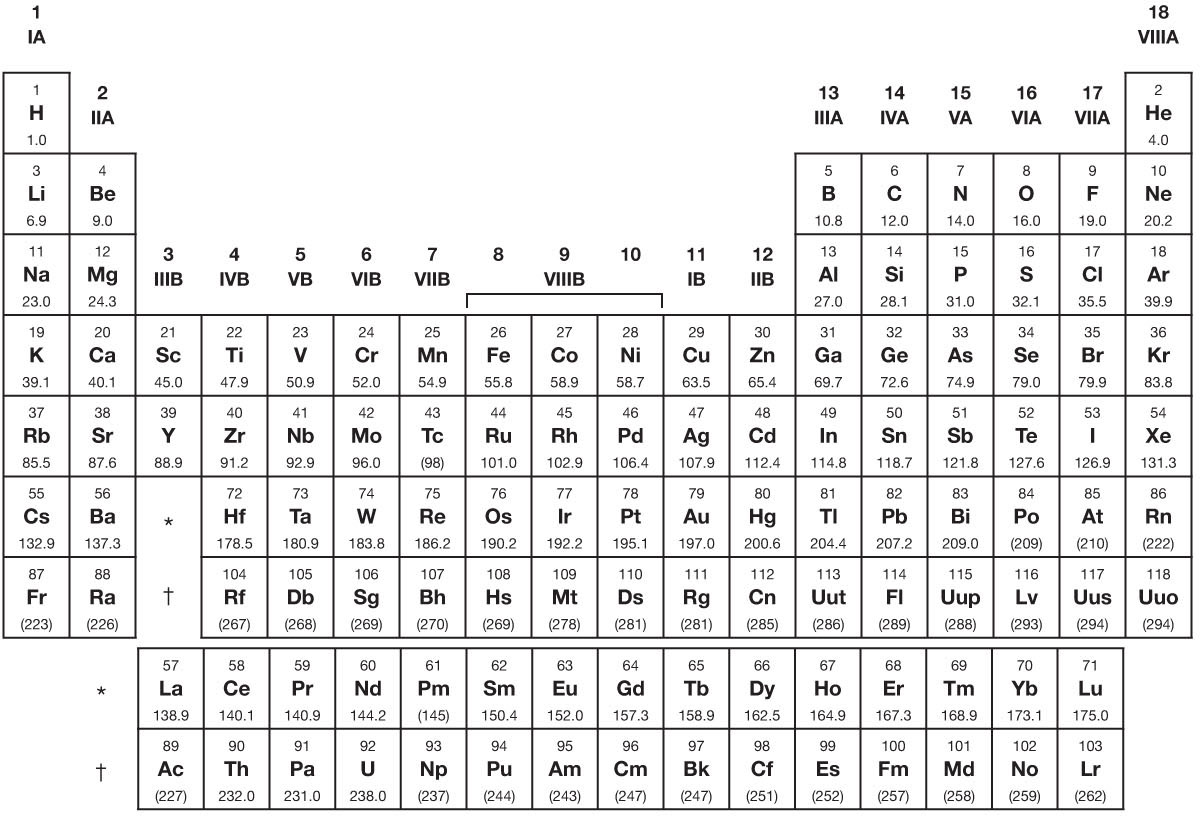
In order to be able to read the Periodic Table of the Elements, there are a few things you need to understand. For starters, elements are arranged in order of increasing atomic number, from left to right and from top to bottom. An element’s atomic number represents the number of its protons (and also the number of its electrons, since those two numbers are the same). The different rows of elements are called periods. The periods correspond to the shells, or the different orbits that electrons occupy around atoms. As a rule, electrons will occupy the lowest shell they can, and move on to higher shells only after lower shells are occupied (although this rule is sometimes violated) so as to minimize the energy of the atom. Every element in the top row (the first period) has one shell for its electrons. Every element in the second row (the second period) has two shells for its electrons, and so on. At this time, the maximum number of shells is seven.
The Periodic Table has a special name for its columns, too. The elements in a column are called a group. The elements in a group have the same number of electrons in their outer shell. It is the group that an element occupies, much more than the period, that determines its chemical properties. This is because the number of electrons needed to complete an element’s outer shell shapes the way in which it reacts with other elements to form molecules. For instance, every element in the first column (Group I) has one electron in its outer shell. The elements in this group are called alkali metals, which describe soft, silvery metals that react strongly with water. The further down the group you go, the more violent this reaction is.
By the same token, every element on the second column (Group II) has two electrons in the outer shell. As you keep counting the columns, you’ll know how many electrons are in the outer shell. Note that the far right-hand column is composed of a group called the noble gases, sometimes referred to as inert gases, because these elements generally don’t react with other elements since their outer shell is completely filled.
Atomic Structure of Lithium and Sodium
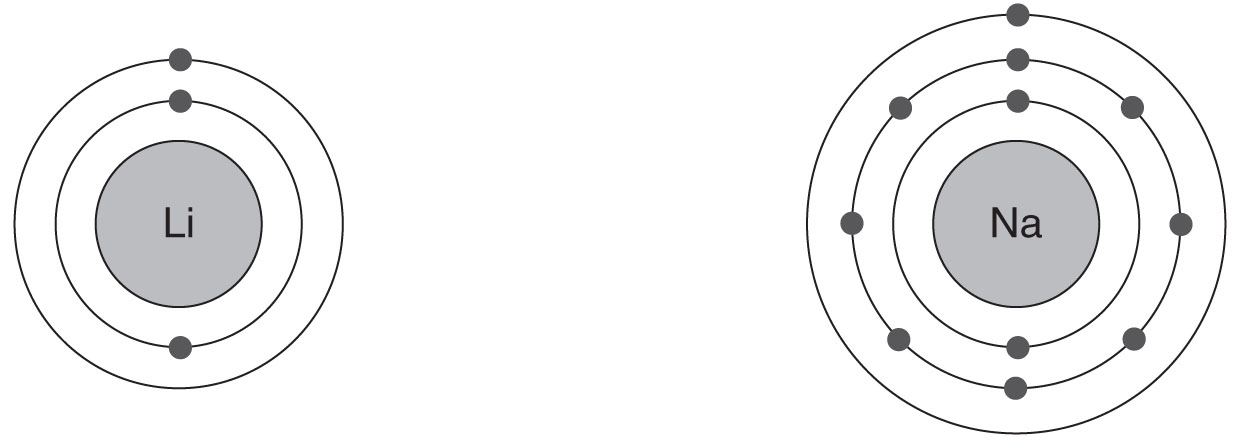
Notice that Li has two electron shells, so it is in the second row of the periodic table; Na has three shells, so it is in the third row. Both elements have one electron in their outer shell and are, therefore, in Group I.
Besides these important chemical properties, an element’s atomic mass is also related to its position on the Periodic Table. The atomic mass listed in an element box represents the average mass of a single atom. Why average? Because some elements can vary in their number of neutrons, so individual atoms of an element may be found in a couple of slightly different sizes. These different sizes are referred to as isotopes.
A pretty good estimate of atomic mass comes from adding the total number of neutrons and protons together. Each proton and neutron has a mass very close to what’s called an atomic mass unit (amu). The majority of hydrogen atoms have only one proton, so its atomic mass should be very close to one, and is, at 1.007 amu.
Now take a look at an individual element box within the Periodic Table. Here’s chlorine.
Chlorine
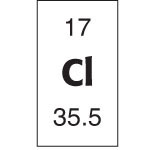
Chlorine’s atomic number is 17, which is the number of protons in the nucleus of a chlorine atom and also the number of electrons orbiting its shells. Its atomic symbol, Cl, is fairly close to its name, unlike the symbols of some other common elements, such as Fe (iron), Au (gold), Ag (silver), W (tungsten), or Na (sodium), symbols that derive from the elements’ Latin names, rather than their familiar English names.
Finally, the atomic mass of chlorine is listed as 35.5, due to about half of chlorine atoms being close to 35 amu and the other half closer to 36 amu. Since all chlorine atoms have 17 protons, this means chlorine atoms are almost as likely to have either 18 or 19 neutrons.
| Question | Analysis |
|---|---|
| How many neutrons does a single sulfur (S) atom have? |
Step 1: The question asks for the number of neutrons in sulfur, which are found in the nucleus of an atom with the protons. |
| Step 2: Since the atomic mass is about equal to the total number of protons and neutrons, and the atomic number is defined by the number of protons, the difference in these values (available on the Periodic Table earlier in this section) should be about equal to the number of neutrons. Round the atomic mass to the nearest whole number as necessary. For sulfur: Atomic # (protons) = 16 Atomic mass = 32.1 Number of protons and neutrons = 32 Number of neutrons = 32 − 16 = 16 |
|
| Step 3: It isn’t possible for an atom to have something other than a whole number of neutrons, so round to the nearest whole number. There are exactly 16 neutrons. | |
| (A) 16 (B) 16.1 (C) 32 (D) 48 |
Step 4: Select choice (A). |
Now try one on your own:
-
How many protons, neutrons, and electrons do argon (Ar) atoms have? - 18, 18, 18
- 18, 22, 18
- 18, 21.9, 18
- 18, 22, 22
Explanation
Answer choice (B) is correct. Argon’s atomic mass is about 40, so there are 18 protons and 22 neutrons, and, in neutral atoms, there are always the same number of electrons as protons.
Compounds
Unstable elements readily form into compounds with properties very distinct from the elements from which they are composed. For instance, sodium and chlorine, both of which are extremely unstable and noxious as elements, combine to form the stable and edible compound sodium chloride (NaCl), more commonly known as table salt. Table salt is called an ionic compound because each chlorine atom borrows an electron from each sodium atom and the atoms stick closely together to form a very tightly bound crystalline structure when salt is in solid form. When it is placed in solution, however, such as when table salt is poured in water, the atoms dissociate into sodium ions and chloride ions. An ion is an electrically charged atom; in this case, each of the sodium atoms is positively charged because it has lent an electron to its corresponding chloride atom, and each of the chloride atoms is negatively charged for the same reason.
Table sugar is an example of a covalent compound, which means, among other things, that it does not ionize when dissolved in water. In a covalent compound, the atoms in the molecule have covalent bonds; that is, they share electrons in pairs, so that each atom provides half the electrons, and the pair is held tightly by both atoms. For this reason, they will not separate as ions do.
Acids and Bases
An acid is a substance that gives up positively charged hydrogen ions (H+) when dissolved in water. Acids corrode metals and generally have a sour taste (though not all acids are safe to drink). Some common acidic solutions are vinegar (which has acetic acid) and lemon juice (which has citric acid). More potent acids include hydrochloric acid, nitric acid, and sulfuric acid (commonly used in lead batteries), all of which are extremely corrosive and must be handled with the utmost care.
A base is a substance that gives up negatively charged hydroxyl ions (OH-) when dissolved in water. Basic substances are also referred to as alkaline. Bases typically taste bitter.
Some common basic substances found in a kitchen include baking soda (sodium bicarbonate) and liquid soap (which often includes potassium hydroxide). More potent bases include sodium hydroxide (also known as lye) and sodium hypochlorite (the most common active ingredient in bleach), and these are just as corrosive and dangerous as comparable acids. When acids and bases react (and they react together rather powerfully), the substances neutralize each other and turn into water and a salt.
The pH of a solution is a number from 0 to 14 that indicates how basic or acidic that solution is. According to the pH scale, solutions with a pH less than 7 are acidic, with the degree of acidity increasing tenfold with each declining number. For instance, black coffee has a pH of 5; vinegar, which is 100 times as acidic as coffee, has a pH of 3; and battery acid, which is 100 times as acidic as vinegar, has a pH of 1. A solution with a pH of 7 is neutral. Pure water has a pH of 7.
Common Substances on the pH Scale
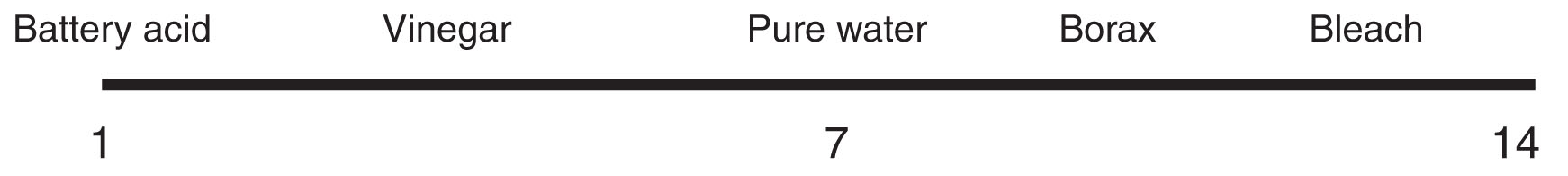
Solutions with a pH greater than 7 are basic, with the degree of alkalinity once again increasing tenfold with each increasing number. Baking soda has a pH that is a little over 8. Borax, commonly used in cleaning solutions and detergents, is about 10 times as basic, at just over 9 on the pH scale, while household bleach, over 12 pH, is 1,000 times as basic as Borax, and 10,000 times as basic as baking soda. Human blood, just above pH 7, is just slightly basic, with about one-tenth the alkalinity of baking soda.
Study this example of an expert test taker’s approach to a question about acids and bases.
| Question | Analysis |
|---|---|
| In a chemistry lab, you are given four solutions and measure their pH values. Which solution contains the greatest number of hydrogen ions? | Step 1: The question asks for the highest concentration of hydrogen ions. |
| Step 2: Acids produce hydrogen ions, and more acidic substances produce them in greater concentrations. Lower pH values indicate more acidic substances, which means the most hydrogen ions. |
|
| (A) the pH 2.5 solution (B) the pH 3.7 solution (C) the pH 7.1 solution (D) the pH 9 solution |
Step 4: Answer choice (A) has the lowest pH. |
Now try your hand at a question about acids and bases.
-
You find a mystery substance in the lab and note the following qualities: it’s bitter to the taste, it releases OH- ions in solution, and it reacts with an acid to form a salt and water. Which of the following could the mystery substance be? - vinegar
- soap
- lemon juice
- water
Explanation
Choice (B) is correct. The properties of the mystery substance match those of a base. Of the choices given, the only basic substance is soap.
Physical Change
Matter may undergo either a physical change or a chemical change. The form, size, and shape of matter may be altered in a physical change, but the molecules remain unchanged. Building a sand castle or bending a piece of plastic into a new shape are both physical changes. The matter has been rearranged, but it is still made of up of the same kinds of atoms and molecules. In other words, its chemical identity has remained intact.
One of the most important kinds of physical change is what’s known as a phase transition. A phase transition is when matter switches from one state of matter, like solid, liquid, or gas, to another.
The solid state exists at lower temperatures relative to the liquid and gaseous states (although the specific temperature ranges depend on the element or compound). In the solid state, atoms or molecules are packed very close together and do not move freely. Solids maintain a constant volume and shape. A good example is an ice cube, consisting of solid-state water molecules.
The liquid state exists at a higher temperature range relative to the solid state equivalent of the same element or compound. In the liquid state, molecules flow freely around each other. Liquids have a constant volume, but do not maintain their shape. They will either take on the shape of their container or, let free, will spread out into a puddle. Water from a faucet comes out in the liquid state.
The gaseous state occurs at a higher temperature range relative to the previous two states of matter. In the gaseous state, molecules flow even more freely from each other, and will also spread out as far as they can. Gases do not maintain either a constant volume or shape. A small amount of gas will spread to fill a large volume if space is available, or can be highly compressed into smaller spaces (oxygen tanks include a large amount of compressed oxygen gas). Steam or vapor are the common terms for water in a gas state.
Under certain circumstances, it’s possible for solids and gases to transition between each other directly, without transforming to the liquid state first. The specific terms for each type of phase transition are summarized below.
Phase Changes
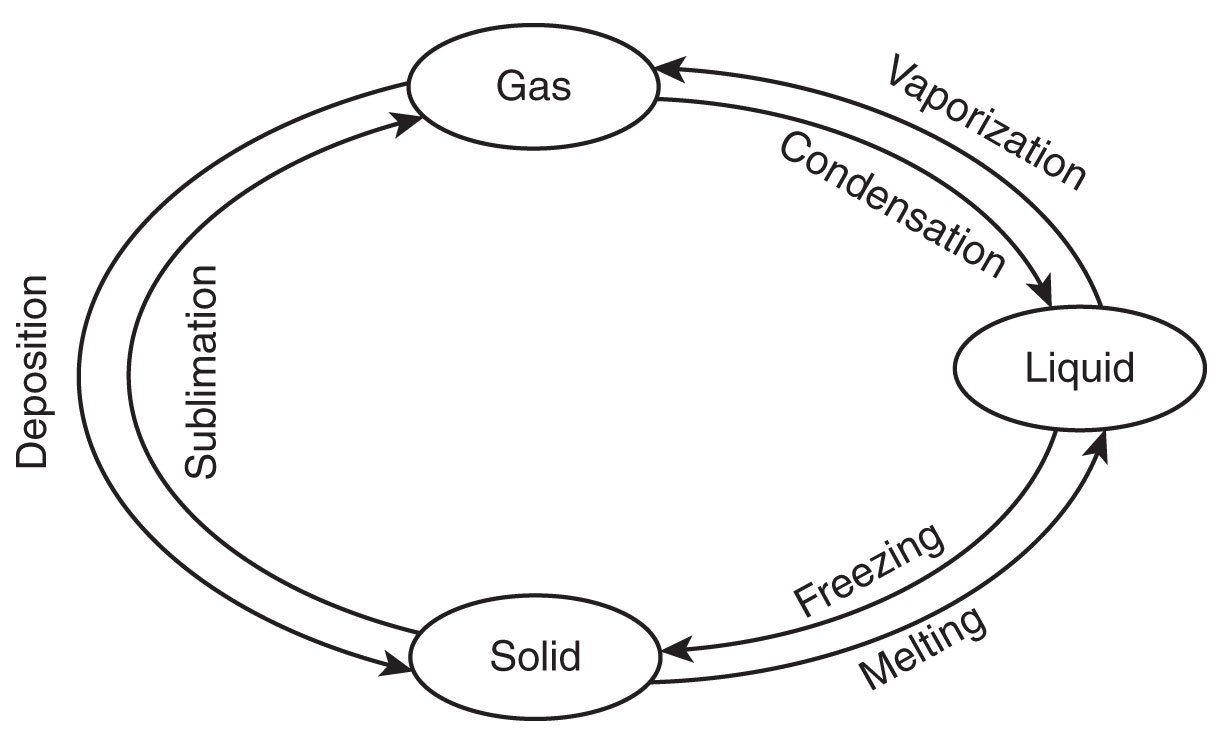
Chemical Change
In a chemical change, molecules of new matter are formed that are different from the original molecules of matter. When elements and compounds undergo a chemical change, the process is called a chemical reaction. During a chemical reaction, atoms are rearranged into new combinations, resulting in different kinds of molecules. The molecules and atoms that enter the reaction are called reactants and the molecules and atoms that result from the reaction are called products.
For example, water is also known as H2O because a single water molecule is made of two hydrogen atoms and one oxygen atom. One kind of chemical reaction will cause many water molecules to break apart and the atoms to rearrange into hydrogen molecules (H2) and oxygen molecules (O2). This is how the reactant, water, is turned into the products, pure hydrogen gas and oxygen gas. Other common examples of chemical reactions include the formation of rust on iron, and the conversion of wood into charcoal, carbon dioxide, and steam in a fire.
To illustrate the profound effects of chemical change, consider one more example: the reaction of chlorine gas and sodium metal. Both substances, in their pure forms, are extremely hazardous to humans. Sodium metal cannot be touched with bare hands; it reacts with any moisture, giving off heat and sparks. It’s stored in oil when not in use. Chlorine gas was used as a devastating chemical weapon in the First World War. Yet, these two dangerous substances can undergo a chemical reaction that results in a product of sodium chloride: common table salt, found in any kitchen. That’s the power of chemical change.
Here’s how the well-prepared test taker would approach a question about states of matter.
| Question | Analysis |
|---|---|
| A sample of matter with an amorphous shape but constant volume has to be | Step 1: The question asks for the type or phase of matter with a constant volume but non-fixed shape. |
| Step 2: A solid has a fixed shape. A liquid will take the shape of its container, or more exactly, find the lowest possible level. | |
| Step 3: The only thing that logically must be true of the matter sample based on the qualities described in the question is that the substance must be in the liquid phase. | |
| (A) in the solid phase (B) in the liquid phase (C) a pure element (D) a compound |
Step 4: Select choice (B). (Note that the sample could be either an element or a compound, since both elements and compounds are capable of being solid, liquid, or gas. Thus, neither (C) nor (D) has to be true.) |
Now try one on your own.
-
Energy is needed for which of the following changes of state? - solid to liquid
- gas to liquid
- liquid to solid
- gas to solid
Explanation
Choice (A) is correct. Liquids occur at higher temperatures than solids, and gases occur at higher temperatures still. The melting of a solid into a liquid is the only answer choice listed that involves movement to a phase that occurs at higher temperatures, requiring a net input of energy to allow the molecules the freer movement that occurs in the liquid phase.