DECAFFEINATION
One of coffee’s foremost properties is that it stimulates the brain and heightens alertness, but these qualities also cause negative effects for some consumers. To counter this problem, various processes have been developed for decaffeinating coffee.
The principle
Caffeine was discovered in 1819 by a German chemist, Friedlieb Ferdinand Runge. And as early as the end of the nineteenth century, research was conducted into ways of limiting its effects or even eliminating it altogether while still preserving the coffee’s other components. Since the time the first method was devised in 1903 by a coffee merchant, Ludwig Roselius, the processes have moved on a great deal, but they are still performed on green coffee beans, making them difficult to roast, and altering their flavors.
Decaffeination using chemical solvents (conventional method)
There are two methods involving the use of solvents.
Direct method
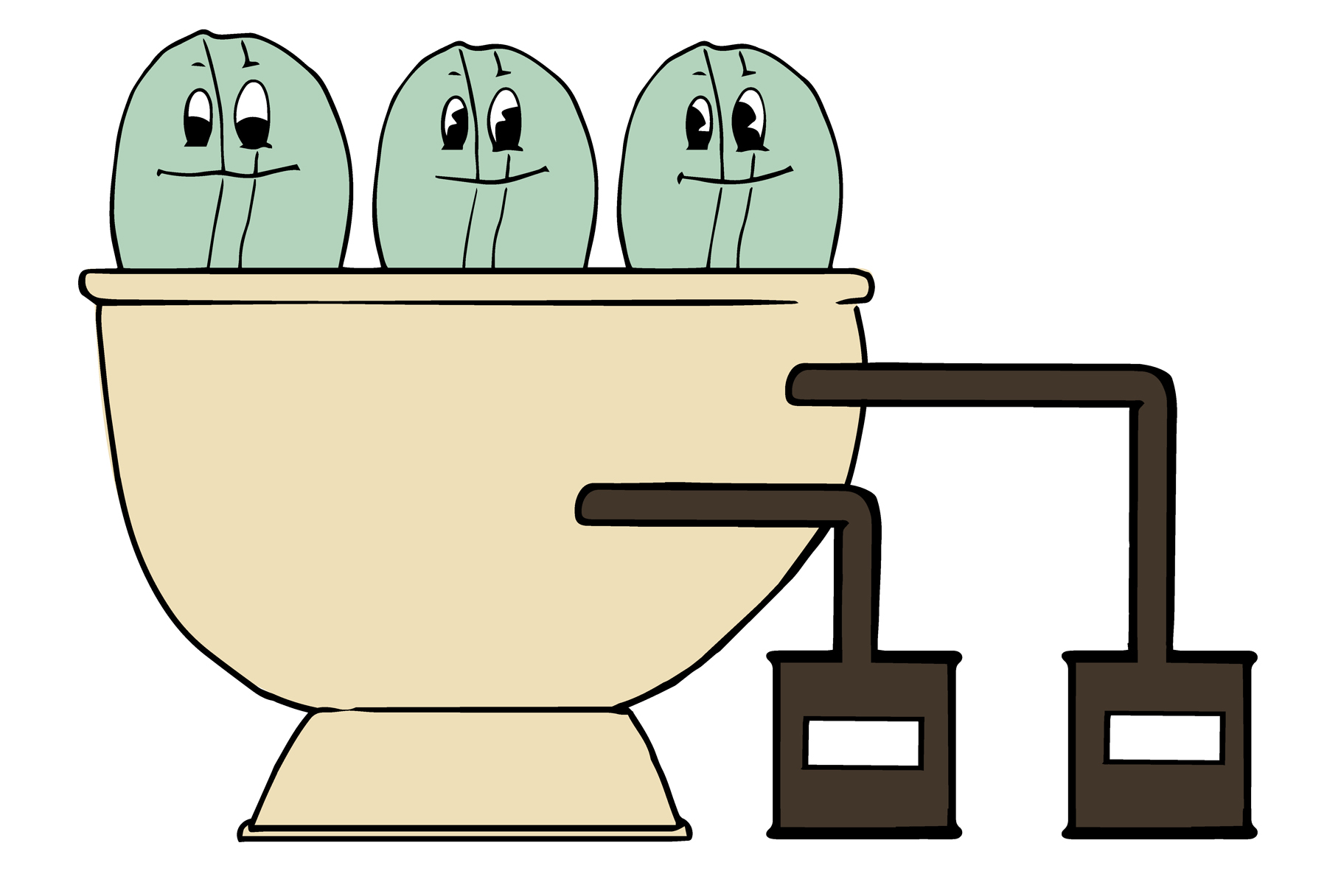
1 The green coffee beans are steamed or immersed in hot water to open their pores.

2 The solvent is added and decaffeination begins.
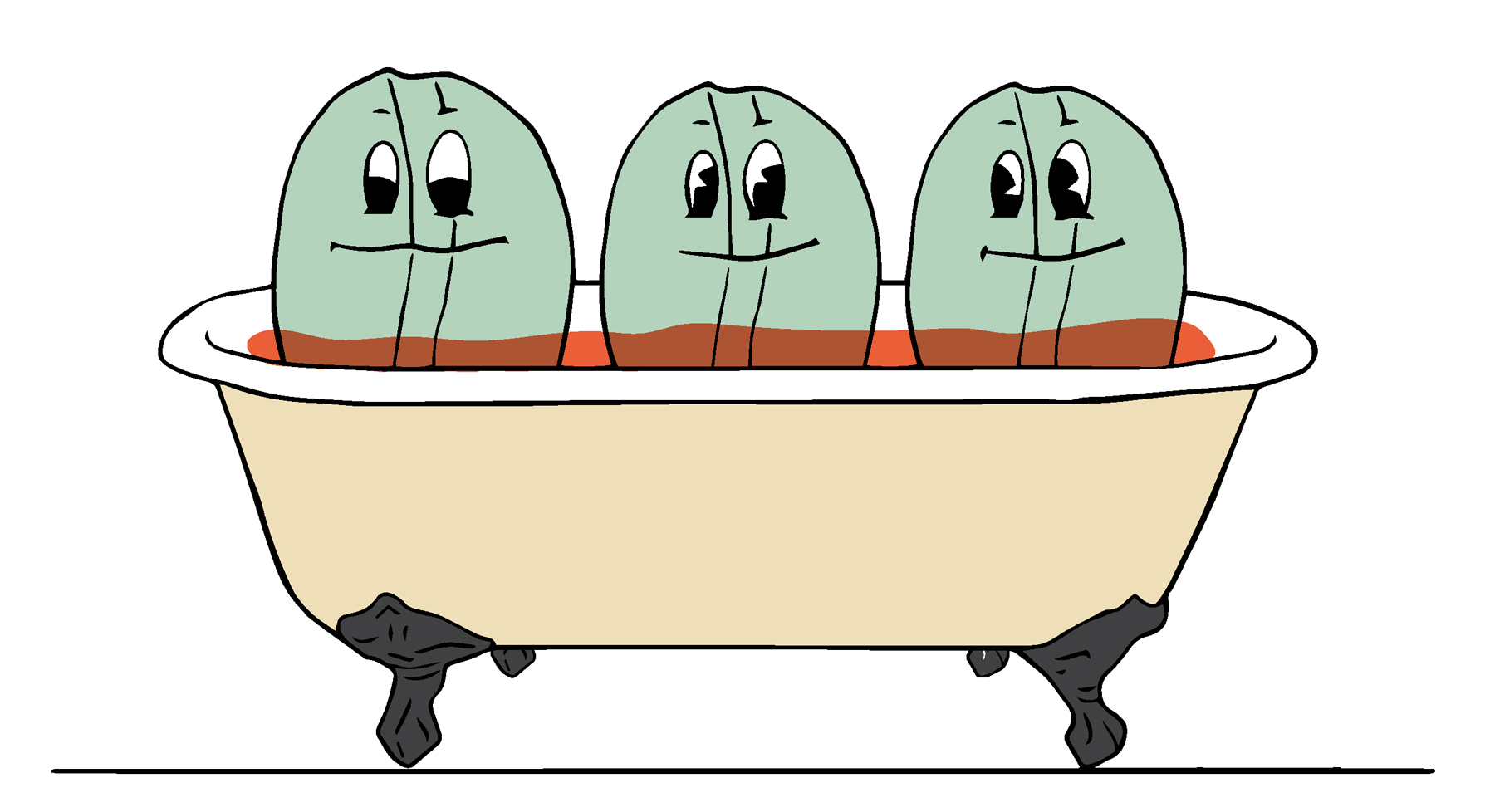
3 The beans are then washed to remove, as far as possible, any traces of solvent.
4 The beans are dried and ready for roasting.
Indirect method
The coffee beans do not come into direct contact with the solvent.
1 The green coffee beans are first immersed in very hot water, which extracts all the soluble elements contained within them.
2 The beans are removed from the water, which is now saturated with these elements, and the water is poured into another tank together with the solvent, which will fix the caffeine.
3 The water is heated to drive off the caffeine-laden solvent by evaporation.
4 The green coffee beans are immersed in the water again, and all the components extracted at the first stage are reintroduced into the beans.
Decaffeination using water: the Swiss Water Process (SWP)
This method does not use chemical solvents. Pioneered in 1933, it was introduced to the market in the 1980s and is patented as the Swiss Water Process.
1 A batch of green coffee beans is immersed in very hot water to extract the caffeine and all the desirable flavor components from them.
2 The water saturated with all these substances is passed through a charcoal filter that captures only the caffeine molecules, which are larger than the others. The batch of green coffee stripped of its caffeine, flavors, and other desirable substances is discarded.

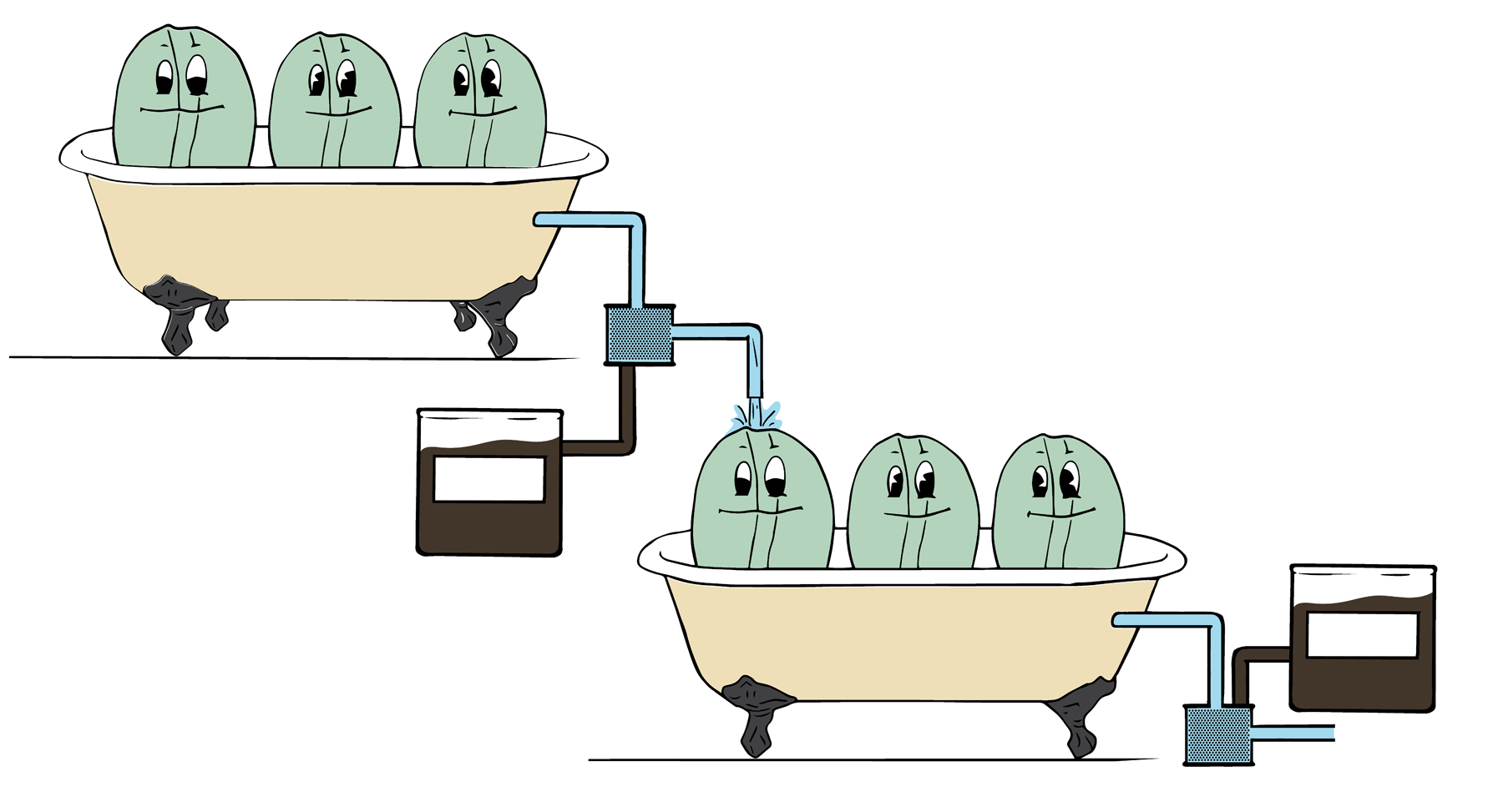
3 A second batch of green coffee is immersed in the water that is carrying the soluble substances from the first batch of green coffee, which is now able to absorb only the caffeine. The flavor components of the second batch will therefore remain in the beans.
4 The water is then refiltered to remove the caffeine so it is ready to receive a third batch of green coffee. The second batch is then dried.
Decaffeination with CO2
This is the newest method. It uses CO2 at a temperature of 88°F and at very high pressure (200 bar), which makes it nearly as dense as water; it is known as supercritical carbon dioxide (CO2).
1 The beans are soaked in water in a tank.
2 Supercritical CO2 is introduced to extract the caffeine. Several cycles are needed for decaffeination to be effective, but this process has the advantage of being more selective than others in its extraction of the caffeine.
3 The caffeine-laden CO2 is then transferred to another container where the pressure is released and the CO2 returns to its gaseous state, leaving the caffeine behind.
4 The decaffeinated coffee beans are dried.