Figure 4.1
Source monitoring as a decision-making model.
4
Psychotic Hallucinations
Hallucinations, especially in the auditory modality (hearing voices), are a common symptom of mental illness and are usually associated with the diagnosis of schizophrenia. Hence various types of hallucinatory experience—for example, audible thoughts or voices heard commenting on one’s actions—were famously included in the German psychiatrist Kurt Schneider’s (1959) list of “first-rank symptoms” of the disorder. Apparently consistent with Schneider’s view, studies have consistently shown that most patients diagnosed as schizophrenic report hallucinatory experiences (Sartorius et al., 1986). For example, in a recent clinical trial conducted with patients suffering from early schizophrenia in the United Kingdom (Tarrier et al., 2004), 177 out of 255 (69 percent) of patients complained of hearing voices. It is sometimes forgotten that this association occurs not because schizophrenia causes hallucinatory voices but because, following Schneider, we define schizophrenia in this way.
Many modern psychopathologists are turning their backs on the schizophrenia concept, arguing that it is too hopelessly vague to be useful clinically or scientifically, that it lumps together patients with diverse problems, and that, as a diagnosis, it does not make useful predictions about the course of illness (whether or not the patient will get better) or which kinds of treatment are most likely to be effective (see Bentall, 2003). Data on hallucinations illustrate some of these difficulties. For example, hallucinations are experienced by approximately 10 percent of patients diagnosed as suffering from bipolar disorder (Goodwin & Jamison, 1990) and also sometimes by patients suffering from major depression (Coryell, 1996; Lattuada, Serretti, Cusin, Gasperini, & Smeraldi, 1999). More surprisingly to many both inside and outside the mental health community, hallucinations are also reported by a sizable minority of individuals who have never had contact with psychiatric services.
The earliest evidence that this was the case emerged from surveys conducted for the purposes of psychical research (Sidgewick, 1894; West, 1948), and similar findings were later reported in studies of selected groups, especially undergraduate students (Barrett & Etheridge, 1992; Bentall & Slade, 1985b). More recently these observations have been supported by data from more carefully conducted epidemiological investigations. For example, in the Epidemiological Catchment Area Study, in which approximately 18,000 people drawn at random from the U.S. population were interviewed about psychiatric symptoms, the annual incidence rate for hallucinations (the numbers experiencing hallucinations during the year of study) was estimated at between 4 and 5 percent, and the lifetime prevalence rate was estimated at between 10 and 15 percent (Tien, 1991). In a study of 7,000 randomly selected Dutch citizens, 7.9 percent had experienced hallucinations (van Os, Hanssen, Bijl, & Ravelli, 2000), and in the more recent U.S. National Comorbidity Study of 8,000 U.S. citizens, 8.5 percent had experienced auditory hallucinations, 7 percent had experienced visual hallucinations, 7 percent had experienced tactile hallucinations, and 1.6 percent had experienced all three kinds (Shevlin, Dorahy, & Adamson, 2007). These figures compare with an estimated lifetime risk of being diagnosed as schizophrenic of about 0.5 percent (Jablensky, 1995; McGrath, 2005). Hence we can roughly estimate that for every schizophrenia patient who hears voices there are about ten voice hearers who do not seek psychiatric treatment.
What are we to make of these findings? One response long favored by psychologists and an increasing minority of psychiatrists has been to view schizophrenia or psychosis (a collective term used to describe all serious mental disorders in which the individual seems, in some sense, to lose touch with reality) as existing on a continuum with normal functioning (Claridge, 1987; van Os et al., 2000). According to this view, the human world is not divided into those who are schizophrenic and those who are not; rather, a continuum runs from ordinary functioning, through frank eccentricity, to full-blown madness. However, given the increasing doubts about the value of categorical diagnoses such as schizophrenia, and given that patients with many diagnoses experience hallucinations, an alternative approach is to try to study and explain hallucinations in their own right.
1 The Phenomenology of Psychotic Hallucinations
A starting point is to understand their phenomenology. A widely cited definition of hallucinations holds that they are percept-like experiences that occur in the absence of appropriate stimuli, which have the full force and impact of corresponding real perceptions and are not amenable to voluntary control (Slade & Bentall, 1988). Since the early nineteenth century, hallucinations have been distinguished from illusions, in which real objects are misperceived (Esquirol, 1832). However, attempts to further subdivide them into different subtypes, for example, a proposal by Jaspers (1913/1963) that we should distinguish between true hallucinations (experienced in external subjective space) and pseudohallucinations (experienced as internally located), have not proved to be enduringly influential. In clinical practice, it is common to encounter patients who experience voices that appear to originate somewhere in the center of the head or sometimes seem to be external to the body and sometimes internal. Detailed analyses of phenomenological data confirm that hallucinatory experiences vary along three relatively independent dimensions: whether they are located internally or externally, whether they are attributed to self or to another agent (some patients report that their voices seem external but that they know that they are self-generated), and whether they are linguistically simple or complex (varying from single words to conversations) (Stephane, 2003). Hallucinated voices also vary in their apparent identity (Leudar, Thomas, McNally, and Glinsky [1997] reported that 64 percent of patients could identify their voice as belonging to a familiar person); the number of voices experienced (usually two or three but sometimes more or less) (Nayani & David, 1996); and their dialogic function (whether they talk about the person, comment on his or her actions, address him or her directly, or issue commands) (Leudar et al., 1997; Nayani & David, 1996).
Reflecting concerns that they may be associated with violence, command hallucinations have received particular attention. In fact, although the majority of psychiatric patients with these kinds of voices sometimes obey them, and although it is not uncommon for voices to urge attacks against other people, it is not clear whether this results in an increased risk of dangerous behavior (Juninger & McGuire, 2001; Kasper, Rogers, & Adams, 1996; McNeil, Eisner, & Binder, 2000). Not surprisingly, the extent to which patients obey hallucinated commands depends not only on the nature of the commands but on beliefs about the voices, with compliance more likely if the voice is believed to be benevolent, authoritative, or uncontrollable (Beck-Sander, Birchwood, & Chadwick, 1997). The extent to which individuals feel subordinate to their voices seems to be closely related to the extent to which they feel subordinate in other social relationships (Birchwood, Meaden, Trower, Gilbert, & Plaistow, 2000). These kinds of beliefs may also help to explain why some people who hear voices seek psychiatric help whereas others do not; a comparison of patients with nonpatient hallucinators found that patients usually believe that they are weaker than their voices, whereas the opposite is the case for the nonpatients (Honig et al., 1998).
When asked, patients tend to explain their judgment that their hallucinated voices are alien in terms of sensory qualities, for example, their apparent clarity, gender (if different to the self), and conversational qualities (Garrett & Silva, 2003). Several decades ago, Aggernaes attempted to define a series of “reality characteristics” that could be used to differentiate hallucinations from other kinds of perceptual experiences (Aggernaes, 1972; Aggernaes, Haugsted, Myschetzky, Paikin, & Vitger, 1976). These included qualities of sensation versus ideation, behavioral relevance, publicness (the extent to which the experience is believed to be shared by others), objectivity (whether the experience occurs across more than one modality), dependence versus independence of a particular mental state, and “involuntarity” (the extent to which it is impossible to dismiss the experience by wishing). When a group who had experienced LSD-induced hallucinations was compared to a group of psychotic patients, the LSD hallucinators were much more likely to report that their experiences changed across these dimensions, but psychotic hallucinations were much less likely to be experienced as independent of the individual (Aggernaes et al., 1976).
2 The Environment and Hallucinations
Research into the causes of psychosis has tended to overemphasize the importance of genetic and endogenous factors (e.g., neuropsychological functioning or imbalances in the brain’s neurotransmitter systems) and has neglected the important role of environmental determinants (Bentall, 2009). Nonetheless the evidence that environmental influences affect the risk of experiencing psychotic symptoms is, if anything, more compelling than the evidence on any specific biological cause and becomes especially consistent when relationships between specific symptoms and specific types of environmental factors are considered (Bentall & Fernyhough, 2008).
Hallucinatory voices tend to be heard under particular environmental conditions. When patients with a history of hallucinating are exposed to carefully controlled environmental conditions, they report that their voices are loudest and most frequent when they are exposed to either sensory restriction (wearing ear mufflers) or when they are exposed to white noise (noise containing a random selection of frequencies; rather like the sound of an untuned radio) (Gallagher, Dinin, & Baker, 1994; Margo, Hemsley, & Slade, 1981). Clinical reports suggest that stress can trigger hallucinations in vulnerable individuals (Siegel, 1984), and indeed, one particular type of stress—bereavement—commonly provokes hallucinations in previously unaffected individuals (Grimby, 1998). In a study in which psychotic patients kept detailed diaries of their experiences, it was found that the onset of hallucinations was preceded by high levels of stress and negative affect (Delespaul, deVries, & van Os, 2002).
Other environmental influences appear to have their affect much earlier in the life histories of people who hear voices. Numerous studies have reported a high prevalence rate of trauma and experiences of abuse among severe mentally ill patients (Goodman, Rosenberg, Mueser, & Drake, 1997; Mueser et al., 1998; Neria, Bromet, Sievers, Lavelle, & Fochtmann, 2002; Read, van Os, Morrison, & Ross, 2005). Similar findings have also been reported in samples of nonpatients (usually students) who score highly on questionnaire measures of psychotic-like experiences (Latasker et al., 2006; Startup, 1999). In a recent meta-analysis which calculated the overall association between childhood trauma and psychosis from all available adequately conducted retrospective, prospective, and epidemiological studies, it was found that a child experiencing trauma (sexual abuse, physical abuse, bullying by peers, or prolonged separation from parents) had an approximately threefold increase in the risk of psychosis, with the risk much greater for those affected by multiple traumas (Varese, Smeets, Drukker, Lieverse, Lataster, Viechtbauer, Read, van Os, & Bentall, 2012).
There is evidence of a specific association between childhood trauma, especially sexual abuse, and hallucinations. A first indication of this association is the similarity between hallucinations and the symptoms of post-traumatic stress disorder (PTSD) (Morrison, Frame, & Larkin, 2003). PTSD is a psychological condition resulting from the exposure to an extreme traumatic stressor and involves three main symptom clusters, usually described as (i) the reexperiencing of symptoms, (ii) the avoidance of trauma-related stimuli, and (iii) hyperarousal (American Psychiatric Association, 2000). The reexperienced symptoms include trauma-related intrusive thoughts, distressing dreams, and dissociative flashback episodes in which the individual vividly reexperiences the triggering event. The perceptual strength and vividness of these symptoms, as well as their intrusiveness and involuntary nature, are consonant with the phenomenological characteristics of psychotic hallucinations discussed earlier. Perhaps unsurprisingly, hallucinations with trauma-related content have been observed both in PTSD patients (Butler, Mueser, Sprock, & Braff, 1996; Hamner et al., 2000; Mueser & Butler, 1987) and in psychotic patients with a history of single or multiple trauma (Hardy et al., 2005; Read & Argyle, 1999).
Patients and nonpatients who hear voices often report that traumatic experiences (or events that reactivate memories of past trauma) directly trigger their first hallucinatory experiences (Honig et al., 1998; Romme & Escher, 1989). A population survey of over 17,000 citizens of California found that a history of childhood trauma was associated with a fivefold increase of experiencing hallucinations, which was independent of the possible confounding effects of substance abuse (Whitfield, Dube, Felitti, & Anda, 2005). Similarly, traumatic childhood experiences were found to be associated with auditory, visual, and tactile hallucinations in an even larger U.S. epidemiological sample (Shevlin et al., 2007). In this study, the probability of experiencing hallucinations was related, in a dose-response way, with the severity of trauma. A study of schizophrenia patients in New Zealand (where psychiatric staff are legally required to inquire about unwanted sexual experiences) found that hallucinations were more closely associated with a history of sexual abuse than any other psychotic symptom. A study of British patients diagnosed as suffering from bipolar disorder reported the same result (Hammersley et al., 2003), indicating that this association transcends traditional diagnostic boundaries. Similar associations between sexual trauma and delusions (the other major symptom of psychotic illness) have not been found (Famularo, Kinscherff, & Fenton, 1992; Hammersley et al., 2003; Sansonnet-Hayden, Haley, Marriage, & Fine, 1987). Indeed, a recent analysis of data from a British epidemiological study (which took into account the fact that hallucinations and delusions sometimes occur together) tested for a specific association between childhood sexual abuse and hallucinations and found it (Bentall, Wickham, Shevlin, & Varese, 2012).
Sometimes psychiatric patients report that the contents of their hallucinations reflect their traumatic experiences (Read, Agar, Argyle, & Aderhold, 2003). In one study of patients diagnosed as suffering from schizophrenia, thematic links were found between the content of hallucinatory voices (e.g., threat, humiliation, guilt) and the experience of past trauma (Hardy et al., 2005). However, a literal correspondence between hallucinatory content and previous trauma was found in only 12.5 percent of patients.
These findings raise two conundrums that challenge the psychopathologist. First, hallucinations usually appear many years after the experience of childhood trauma, and any comprehensive explanatory model will therefore have to explain this gap. Second, why is it that although trauma is causally implicated in hallucinations, content does not always exactly reflect the traumatizing event? We will return to these questions after first reviewing current thinking about the psychological mechanisms underlying hallucinations.
Psychotic hallucinations have, at one time or another, been attributed to a variety of neurobiological and psychological processes, including conditioning processes, abnormal mental imagery, and the release of preconscious mental contents into consciousness, but the evidence base for these accounts has often been flimsy (Slade & Bentall, 1988). However, in the last two decades, a consensus has emerged that hallucinations are the consequence of the failure to discriminate between internally generated mental events and events in the external world (Bentall, 1990; Frith, 1992; Hoffman, 1986; Laroi & Woodward, 2007). Although different investigators have proposed different versions of this model, they all assume that auditory-verbal hallucinations occur when inner speech is misattributed to a source that is external or alien to the self. Thus hallucinations are said to result from a failure of “source monitoring.”
It will be helpful at this point to define what is meant by the term “inner speech.” Most if not all of us are aware of inner dialogue, a silent voice with which we debate what is happening to us, make plans and decide what to do, and sometimes use to chastise ourselves when things go wrong. This process has been most extensively studied by developmental psychologists following a celebrated debate between the Swiss pioneer of child psychology Jean Piaget (1926) and his Russian contemporary Lev Vygotsky (1962). Noticing that young children often speak aloud to themselves, Piaget argued that they do so because they are “egocentric” and unaware that no one is listening. Vygotsky, on the other hand, argued that this stage is part of the pathway between the animal-like thinking processes of the preverbal child and the verbal thought of the adult. According to Vygotsky, children first learn social speech in the company of caregivers and then discover that, in addition to issuing and responding to verbal directions in conversations with others, they can issue and respond to commands in conversations with themselves. Hence “private speech” (speech that is self-directed but audible to others) is functional and becomes established as an important method of self-regulation. Later, according to Vygotsky, the child learns to use self-regulatory speech covertly, at which point private speech becomes inner speech. Eventually inner speech becomes telegraphic and quite distinct from social speech.
The role of inner speech in human mental life has been neglected by cognitive psychologists in recent decades. However, developmental research, especially a series of landmark studies by Kohlberg, Yaeger, and Hjertholm (1968), definitively resolved the debate about its function in Vygotsky’s favor (Berk, 1994). Furthermore, beginning as early as the 1930s, electromyographical studies, in which muscular activity was measured during thinking, demonstrated that even during adult inner speech, covert activations of the speech muscles could be detected (McGuigan, 1978). It seems that when we think in words, our speech muscles “light up.” (Of course, this does not mean that we need our speech muscles to think; rather, this is a neuromuscular echo of a time during childhood when we could only think in words by speaking.) More recent studies, using the new neuroimaging technologies such as functional magnetic resonance imaging (fMRI), have shown that inner speech is associated with activations of both the speech generation and language processing areas, which (in most people) are localized in the left cerebral cortex (Jones & Fernyhough, 2007). A particularly interesting series of studies conducted by Ford and Mathalon (2004), who assessed the coherence of electroencephalography (EEG) signals from different brain regions, found that during the production of normal inner speech, corollary signals from the speech generation areas in the frontal cortex suppress the activation of the more posterior language processing areas. Crudely, it might be said that when we talk to ourselves, the frontal language production areas of the brain tell the posterior areas not to bother listening.
Studies that have demonstrated that manifestations of inner speech coincide with self-reports of verbal hallucinations provide the primary evidence for the source monitoring model. In a remarkable series of early investigations, Gould (1948, 1949, 1950) showed not only that verbal hallucinations were accompanied by increased speech muscle activity as recorded by electromyography (EMG) but also (in the case of one unusual patient who whispered while hearing voices) that he could use a sensitive throat microphone to record actual hallucinations. A similar case study was later reported by Green and Preston (1981). Subsequent EMG studies broadly replicated Gould’s observations (Inouye & Shimizu, 1970; McGuigan, 1966).
In recent years, researchers have attempted to use more sophisticated technologies such as positron emission tomography (PET), EEG, and fMRI to directly visualize activations in the brain as patients hallucinate. However, the results have been complex and sometimes difficult to interpret. An early study by McGuire, Shah, and Murray (1993), using PET, reported that auditory hallucinations were associated with an increase in blood flow to Broca’s area, a region in the left (in most right-handed individuals) frontal lobe that has long been associated with speech production. This finding seemed to support the notion that auditory hallucinations are misattributed inner speech. However, subsequent studies sometimes implicated the left frontal cortex (Shergill, Bullmore, Simmons, Murray, & McGuire, 2000), sometimes did not (Silbersweig et al., 1995), and often detected a wide range of cortical and subcortical activations accompanying hallucinated speech (Allen, Aleman, & McGuire, 2007), particularly activations in the left temporal structures involved in speech perception (Allen, Amaro, et al., 2007; Dierks et al., 1999).
It seems likely that the inconsistencies in the neuroimaging data reflect both methodological challenges and oversimplistic thinking about the nature of inner speech. In a typical fMRI study, for example, patients are asked to press a button when they hear a voice, and neural activations are matched to the time course of button presses. However, few patients are able to do this (many have hallucinations that are continuous, and others hear voices that are too unpredictable), and the blood-oxygenated hemoglobin response measured by fMRI has a slow time course, taking about five seconds to reach full strength, making it difficult for researchers to match data to the onset of hallucinations. Whereas it is often assumed that inner speech corresponds simply to a covert verbal motor response, as conceived by early behaviorists, mature inner speech may involve a much more complex internal representation of speech activity (Jones & Fernyhough, 2007). It is also sometimes assumed that inner speech is absent when patients are not hallucinating, whereas, of course, this is unlikely to be the case. In recognition of these factors, some researchers have used fMRI protocols in which patients have been asked to generate inner speech or to discriminate between their own speech and the speech of others (source monitoring) in the hope of identifying brain regions involved in this kind of discrimination (Allen, Amaro, et al., 2007; Shergill et al., 2000; Sritharan et al., 2005). Others have observed that the interconnectivity of different brain regions may be particularly important when attempting to understand the neural substrate of hallucinations. Using EEG, Ford and Mathalon (2004) found that patients with hallucinations, in comparison with controls, showed a decreased corollary signal from the frontal speech production areas to the auditory cortex.
Clearer evidence in support of the source monitoring model has been obtained from studies that have used psychological methods to measure the ability to distinguish between self-generated and externally generated cognitive contents. Three different experimental procedures have been used for this purpose: (i) the signal detection paradigm, (ii) the source monitoring paradigm, and (iii) the self-monitoring paradigm.
Signal detection theory (SDT) is commonly used to investigate the capacity of individuals to detect stimuli against noisy backgrounds. This approach assumes that this capacity is influenced by two parameters: perceptual sensitivity (d′), which corresponds to the capacity to accurately detect a signal when it is present, and response bias (β), which indicates the individual’s willingness to assume that a signal is present under conditions of uncertainty. The idea here is that the observed pattern of responding will reflect the combination of these two parameters. If d′ is optimum, stimuli will be correctly detected with few false alarms. A shift in β, by contrast, will lead to an increase in hits, but only at the cost of an increase in false alarms (think of a cold war radar operator waiting to scramble jet interceptors who, while looking at the noisy radar screen, believes that the enemy may attack at any moment).
Bentall and Slade (1985a) first used a SDT task to investigate differences in performance between patients with and without hallucinations, and between students scoring high and low on a questionnaire measure of hallucination proneness. Participants were asked to listen to recordings of white noise (unpatterned stimulation rather like the sound from a badly tuned radio) in which, sometimes, a voice was presented; the participants then indicated when they believed they had detected the voice. The hallucinating patients had a greater bias toward detecting signals when compared to nonhallucinating patients, but showed no difference in perceptual sensitivity, and the same pattern of results was found when the hallucination-prone students were compared to those scoring low in hallucinatory predisposition. These results were later replicated in other nonclinical studies adopting similar methodologies (Barkus, Stirling, Hopkins, McKie, & Lewis, 2007; Rankin & O’Carrol, 1995) and in psychiatric patients using slightly different approaches (Vercammen, de Haan, & Aleman, 2008). In a recent study with students, we have found that impaired signal detection performance is specifically related to hallucination disposition, and not to paranoid thinking or nonhallucinatory intrusive thoughts, though the disposition to hallucination, paranoid thinking, and intrusive thoughts were all highly correlated (Varese, Barkus, & Bentall, 2011b). Taken as a whole, the results from these studies demonstrated that patients with auditory hallucinations showed a marked tendency to report false alarms under conditions of uncertainty.
The source monitoring paradigm differs from the SDT approach, because participants are asked to distinguish between memories of self-generated events (typically thoughts) and memories of heard material (Johnson, Hashtroudi, & Lindsay, 1993). In the typical source monitoring task, participants are provided with a list of cue words read out loud by the experimenter. For each word, participants are required to generate a word associated with the cue. After a delay, participants are asked to complete a word recognition task including the words generated by the participant, the words presented by the experimenter, and new words. Participants are therefore requested to indicate whether the word presented is old or new, and whether it was self-generated or was read out by the experimenter.
In the earliest source monitoring study with psychotic patients (Bentall, Baker, & Havers, 1991), hallucinating patients, patients with paranoid delusions but without hallucinations, and healthy participants were presented with cues varying in the level of cognitive effort required to produce an associate (e.g., “Think of a vehicle beginning with C”; “Think of a vegetable beginning with O”; ordinary people typically find the first of these questions easier than the second). Participants were also presented with a series of paired associates similarly varying in difficulty (e.g., “dwelling–house” is easy, whereas unless one lives in Scandinavia, “country–Norway” is hard). A week later they were presented with a list of words including their responses to the cues (“car,” “onion”), the associates produced by the experimenter (“house,” “Norway”), and words that had not previously been presented, and were asked to classify them as self-generated, presented by the experimenter, or new. Overall, the results of this study indicated that words are more likely to be correctly recognized as self-generated if they required cognitive effort (that is, by recalling the cognitive effort involved, we recognize that a word is self-generated). However, the hallucinating patients seemed to be unable to benefit from this clue and hence were more likely than the controls to misattribute high-cognitive-effort self-generated words to the experimenter.
In a series of studies, Brebion and his colleagues investigated the relationship between source monitoring performance of patients with diagnosis of schizophrenia and specific symptoms (Brebion, Amador, David, Malaspina, & Sharif, 2000; Brebion et al., 1999; Brebion, David, Jones, & Pilowski, 2005; Brebion, Gorman, Amador, Malaspina, & Sharif, 2002). While source monitoring perturbation involving the attribution of self-generated items to an external source seemed to be a general characteristic of patients with a diagnosis of schizophrenia presenting with positive symptoms (Brebion et al., 1999), some of the findings indicated that external misattribution errors were more frequently associated with hallucinations than with other positive symptoms such as delusions and thought disorder (Brebion et al., 2000). Broadly similar results were obtained in studies carried out by Keefe and colleagues (Keefe, 2002; Keefe, Arnold, Bayen, & Harvey, 1999; Keefe, Krabbendam, Dautzenberg, Jolles, & Merckelbach, 2005) and by Brunelin et al. (2006). Poor source monitoring was also shown to be associated with hallucination proneness in a study with students (Laroi, van der Linden, & Marczewski, 2004). In this last study, the hallucination-prone students made more source monitoring errors than the controls, especially on high-cognitive-effort items (replicating the earlier findings of Bentall et al., 1991) and also when the words were emotionally salient.
The third psychological paradigm employed in studies of hallucinations has involved the direct measurement of the online monitoring of self-generated speech (Allen et al., 2004; Johns, Gregg, Allen, & McGuire, 2006; Johns & McGuire, 1999; Johns et al., 2001). In a typical experiment, participants are asked to pronounce out loud a list of words into a microphone. Certain auditory features of the participants’ speech, for example, pitch, are then manipulated, and the speech is played back to the participants. At various points in the experiment, the participants are presented with someone else’s prerecorded voice pronouncing the same word. After each trial, participants are requested to identify the correct source of the auditory feedback.
Johns and colleagues (1999, 2001) found that hallucinating patients with diagnosis of schizophrenia were more likely to judge their own speech as alien than both psychiatric and nonclinical controls, especially when the words were derogatory rather than neutral or complimentary, a finding that has been replicated in some subsequent studies (e.g., Allen et al., 2004). Against these findings, one study using this technique found that poor self-speech recognition was associated with acute psychosis and not specifically with hallucinations (Johns, Gregg, Allen, Vythelingum, & McGuire, 2006), and another reported no evidence of impaired self-speech recognition in patients (Versmissen et al., 2007).
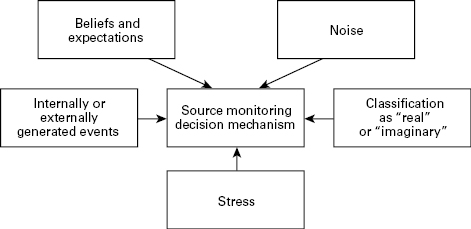
Overall, and despite some inconsistencies, the evidence we have considered seems to support the hypothesis that source monitoring is impaired in people suffering from hallucinations. Indeed, in a recent meta-analysis of published data from the three paradigms, we have found consistent and strong evidence that hallucinating patients perform poorly on source monitoring tasks compared to nonhallucinating patients (Brookwell, Bentall, & Varese, in press). On this view, hallucinations might be understood within a decision-making framework. According to this framework (see fig. 4.1), we are all constantly making decisions (albeit unconscious ones) about whether our experiences are internally generated (“thoughts,” “imaginations”) or externally generated, and hallucinating people differ from nonhallucinating people in the way in which they make these decisions. This model helps us to understand some of the observations that we have considered earlier.

Figure 4.1
Source monitoring as a decision-making model.
It is perhaps unsurprising that hallucinations are most often heard under conditions of restricted or unpatterned stimulation (Gallagher et al., 1994; Margo et al., 1981), because it is in precisely these conditions that individuals would be expected to adopt a relaxed criterion for deciding that an apparent stimulus is “real.” Stress might affect the source monitoring process by affecting processing and leading to hasty decision making. The impact of beliefs and expectations on source monitoring might explain why hallucinations are more common in some cultures than others (Al-Issa, 1995); in fact, we have shown experimentally that hallucinating patients are highly influenced by suggestions when making source monitoring judgments (Haddock, Slade, & Bentall, 1995). That hallucinating patients seem to have particular difficulty using cognitive effort as a cue (Bentall et al., 1991; Laroi et al., 2004) is possibly consistent with Ford and Mathalon’s (2004) observation of impaired corollary discharge from the speech production areas in patients with auditory hallucinations.
Several unresolved issues remain. The first concerns whether poor source monitoring is a state marker of particular episodes of hallucination or a vulnerability marker that precedes the onset of voices. In an attempt to resolve this issue Brunelin et al. (2007) compared the source monitoring performance of patients who took part in their previous studies with new data from healthy siblings of patients with diagnosis of schizophrenia. The patients’ siblings were found to perform worse than healthy controls on a source monitoring task, but better than nonhallucinating patients, with hallucinating patients performing the worst of all. On the basis of these findings, the researchers suggested that source monitoring deficits are a genetically inherited trait vulnerability marker for psychosis. More recently, using a signal detection paradigm, we found no difference in source monitoring between currently hallucinating patients and patients who had hallucinated in the past, although both groups showed impaired source monitoring compared to both patients who had never hallucinated and to nonpsychiatric controls, again suggesting that impaired source monitoring is a vulnerability factor that is stable over time (Varese, Barkus, & Bentall, 2011a).
The second unresolved issue, which we address shortly, is why some types of mental contents, but not others, are misattributed to an external agent. Hallucinating patients certainly do not seem to doubt that they think; hence many, perhaps most, of their thoughts are correctly attributed to their own agency. Only some thoughts, particularly emotionally charged ones, tend to get misattributed.
5 Why Does Trauma Cause Hallucinations?
We end this chapter with some speculation about the relationship between early sexual trauma and hallucinations. This relationship, which appears to be fairly specific (in the sense that sexual trauma does not seem to be so closely associated with other psychotic symptoms, although it does, of course, often lead to a wide range of psychiatric difficulties outside the psychotic domain; see Paolucci, Genuis, & Violato, 2001; Putnam, 2003), is presumably mediated by the cognitive mechanisms underlying hallucinations. Hence by understanding this relationship, we should be able to cast further light not only on the way in which adversity leads to psychiatric distress but also on the mental processes that lead to the misattribution of mental events to external causes.
As Morrison has pointed out (Morrison, 2001; Morrison & Baker, 2000; Morrison et al., 2003), and as we have already discussed, the most obvious common feature of hallucinations and nonpsychotic reactions to trauma (especially the reexperiencing symptoms of PTSD) is the intrusion of unwanted thoughts into consciousness. Thus the simplest model of the trauma-hallucination link would assume that impaired source monitoring is a vulnerability factor for hallucinations, which only causes the misattribution of mental events to external causes in the case of certain kinds of mental events. The candidate events would, of course, be post-traumatic intrusive thoughts. This model appears to make sense because research with ordinary people shows that low-cognitive-effort thoughts (which equate to thoughts that come unbidden) are more difficult to source monitor than high-cognitive-effort thoughts (Johnson et al., 1993). It need not be assumed, according to this model, that the misattributed thoughts would have to be directly about the trauma, as, depending on circumstances, PTSD victims tend to be anguished by a range of unpleasant thoughts and ideas. For example, if they feel at least partially responsible for the traumatic event (a not uncommon occurrence in the case of childhood sexual abuse), they may be tormented by thoughts of shame and worthlessness (Feiring, Taska, & Lewis, 2002; Wiffen & MacIntosh, 2005).
In favor of this model, hallucinated patients often report a high level of intrusive negative thoughts (Morrison & Baker, 2000). Furthermore, in common with patients suffering from PTSD and other nonpsychotic disorders marked by intrusive thinking (e.g., obsessive compulsive disorder [OCD]), patients and nonpatients suffering from hallucinations often report metacognitive beliefs (beliefs about their own cognitive processes) that reflect catastrophic fears about the controllability of their own thinking processes (Garcia-Montes, Cangras, Perez-Alvarez, Fidalgo, & Gutierrez, 2006; Laroi et al., 2004; Lobban, Haddock, Kinderman, & Wells, 2002; Morrison & Petersen, 2003; Morrison & Wells, 2003).
In our recent work, we have begun to evaluate this model by exploring the associations between the relevant cognitive processes. In a study with students (Varese, Barkus, & Bentall, 2011b), we found a high correlation between self-reported intrusive thoughts and scores on a widely used hallucination questionnaire. However, against our expectations, we were able to identify a small subgroup of students who scored highly on hallucination proneness but low on intrusive thinking. When high-hallucination, high-intrusive-thinking students were compared to students who scored highly on hallucinations but not on intrusive thinking, with a third group who scored highly on intrusive thinking but not hallucination proneness, and with a fourth group who scored low on both measures, we found that signal detection performance was specifically linked to hallucination proneness and not intrusive thinking, whereas abnormal metacognitive beliefs were linked specifically with intrusive thinking and not hallucinations. This finding, which obviously needs to be replicated in a study of patients, can be interpreted within a two-hit model in which source monitoring impairment, perhaps a consequence of some kind of neurobiological abnormality, is a vulnerability factor for the occurrence of hallucinations, and trauma-induced intrusive thoughts are a contributory factor that increases the risk that hallucinations will be experienced. This account assumes that hallucinations are possible in the absence of trauma if source monitoring is sufficiently impaired.
However, a further observation from this study raised the possibility that trauma might lead to hallucinations by a quite different mechanism. We administered a questionnaire measure of mindfulness (roughly, the ability to be in the present moment; see Bishop et al., 2004) and found that this was closely related to hallucination proneness and not intrusive thoughts. The significance of this is that mindfulness lies at the opposite end of a spectrum of awareness from dissociation (Walach, Buchheld, Buttenmuller, Kleinknecht, & Schmidt, 2006), a state of mind in which complex activities are carried out apparently without awareness (the most familiar example, perhaps, is when one drives a long distance while being emotionally preoccupied, and therefore without being aware of the journey). Dissociation, in turn, although not included in the diagnostic criteria for PTSD, is a common consequence of childhood trauma (Sanders & Giolas, 1991). Hence the possibility is raised that dissociation or lack of mindfulness (which, at the cognitive level, would reflect a subtle failure of attention) affects the source monitoring process.
To test this possibility, we recently carried out a study in which we measured dissociative experiences in patients experiencing hallucinations, patients who had hallucinated in the past, patients who had never hallucinated, and nonpsychiatric controls (Varese, Barkus, & Bentall, 2011a). As noted earlier, in this study, impaired signal detection scores were found in both current and past hallucinators, who performed less well than both the never-hallucinated patients and the healthy controls. Dissociation scores were unrelated to signal detection but were especially elevated in the currently hallucinated patients. Interestingly, statistical analysis revealed that reports of childhood sexual abuse in the sample predicted dissociation scores. These findings can also be interpreted as consistent with a two-hit model in which poor source monitoring is a vulnerability factor that likely predates the onset of hallucinations, but in this account dissociation, caused by early childhood trauma, is the second contributory factor.
The two hypotheses we are offering here for the association between childhood trauma and psychosis are not necessarily mutually exclusive. Childhood trauma no doubt leads both to intrusive thoughts which are difficult to source monitor, and also to dissociative states which affect source monitoring. We hope that these hypotheses provide an agenda for further research. There is a great need for further studies to investigate precisely how different kinds of adversity, at different stages of development, impact on cognitive and neurobiological processes, leading to an increased risk of mental illness in adult life.
References
Aggernaes, A. (1972). The experienced reality of hallucinations and other psychological phenomena: An empirical analysis. Acta Psychiatrica Scandinavica, 48, 220–238.
Aggernaes, A., Haugsted, R., Myschetzky, A., Paikin, H., & Vitger, J. (1976). A reliable clinical technique for investigation of the experienced reality and unreality qualities connected with everyday life experiences in psychotic and non-psychotic persons. Acta Psychiatrica Scandinavica, 53, 241–257.
Al-Issa, I. (1995). The illusion of reality or the reality of an illusion: Hallucinations and culture. British Journal of Psychiatry, 166, 368–373.
Allen, P., Aleman, A., & McGuire, P. K. (2007). Inner speech models of auditory verbal hallucinations: Evidence from behavioural and neuroimaging studies. International Review of Psychiatry, 19, 407–415.
Allen, P., Amaro, E., Fu, C. H. Y., Williams, S. C. R., Brammer, M. J., & Johns, L. C. (2007). Neural correlates of the misattribution of speech in schizophrenia. British Journal of Psychiatry, 190, 162–169.
Allen, P., Johns, L. C., Fu, C. H. Y., Broome, M. R., Vythelingum, G. N., & McGuire, P. K. (2004). Misattribution of external speech in patients with hallucinations and delusions. Schizophrenia Research, 69, 277–287.
American Psychiatric Association. (2000). Diagnostic and statistical manual for mental disorders (4th Ed., text revision). Washington, DC: American Psychiatric Association.
Barkus, E., Stirling, J., Hopkins, R., McKie, S., & Lewis, S. (2007). Cognitive and neural processes in non-clinical auditory hallucinations. British Journal of Psychiatry, 191(suppl. 51), 76–81.
Barrett, T. R., & Etheridge, J. B. (1992). Verbal hallucinations in normals: I. People who hear voices. Applied Cognitive Psychology, 6, 379–387.
Beck-Sander, A., Birchwood, M., & Chadwick, P. (1997). Acting on command hallucinations: A cognitive approach. British Journal of Clinical Psychology, 36, 139–148.
Bentall, R. P. (1990). The illusion of reality: A review and integration of psychological research on hallucinations. Psychological Bulletin, 107, 82–95.
Bentall, R. P. (2003). Madness explained: Psychosis and human nature. London: Penguin.
Bentall, R. P. (2009). Doctoring the mind: Why psychiatric treatments fail. London: Penguin.
Bentall, R. P., Baker, G. A., & Havers, S. (1991). Reality monitoring and psychotic hallucinations. British Journal of Clinical Psychology, 30, 213–222.
Bentall, R. P., & Fernyhough, C. (2008). Social predictors of psychotic experiences: Specificity and psychological mechanisms. Schizophrenia Bulletin, 34, 1009–1011.
Bentall, R. P., & Slade, P. D. (1985a). Reality testing and auditory hallucinations: A signal-detection analysis. British Journal of Clinical Psychology, 24, 159–169.
Bentall, R. P., & Slade, P. D. (1985b). Reliability of a measure of disposition towards hallucinations. Personality and Individual Differences, 6, 527–529.
Bentall, R. P., Wickham, S., Shevlin, M., & Varese, F. (2012). Do specific life adversities lead to specific symptoms of psychosis? A study from the 2007 The Adult Psychiatric Morbidity Survey. Schizophrenia Bulletin, 38, 661–667.
Berk, L. E. (1994). Why children talk to themselves. Scientific American, 271, 78–83.
Birchwood, M., Meaden, A., Trower, P., Gilbert, P., & Plaistow, J. (2000). The power and omnipotence of voices: Subordination and entrapment by voices and significant others. Psychological Medicine, 30, 337–344.
Bishop, S. R., Lau, M., Shapiro, S., Carlson, L., Anderson, N. D., Carmody, J., et al. (2004). Mindfulness: A proposed operational definition. Clinical Psychology: Science and Practice, 11, 230–241.
Brebion, G., Amador, X., David, A., Malaspina, D., & Sharif, Z. (2000). Positive symptomatology and source monitoring failure in schizophrenia: An analysis of symptom-specific effects. Psychiatry Research, 95, 119–131.
Brebion, G., Amador, X., Smith, M. J., Malaspina, D., Sharif, Z., & Gorman, J. M. (1999). Opposite links of positive and negative symptomatology with memory errors in schizophrenia. Psychiatry Research, 88, 15–24.
Brebion, G., David, A. S., Jones, H., & Pilowski, L. S. (2005). Hallucinations, negative symptoms, and response bias in a verbal recognition task in schizophrenia. Neuropsychology, 5, 612–617.
Brebion, G., Gorman, J. M., Amador, X., Malaspina, D., & Sharif, Z. (2002). Source monitoring impairments in schizophrenia: Characterization and association with positive and negative symptomatology. Psychiatry Research, 112, 27–39.
Brookwell, M. L., Bentall, R. P., & Varese, F. (in press). Externalizing biases and hallucinations in source monitoring, self-monitoring, and signal detection studies: A meta-analytic review. Psychological Medicine.
Brunelin, J., Combris, M., Poulet, E., Kallel, L., D’Amato, T., Dalery, J., et al. (2006). Source monitoring deficits in hallucinating compared to non-hallucinating patients with schizophrenia. European Psychiatry, 21, 259–261.
Brunelin, J., D’Amato, T., Brun, P., Bediou, B., Kallel, L., Senn, M., et al. (2007). Impaired verbal source monitoring in schizophrenia: An intermediate trait vulnerability marker? Schizophrenia Research, 81, 41–45.
Butler, R. W., Mueser, K. T., Sprock, J., & Braff, D. L. (1996). Positive symptoms of psychosis in posttraumatic stress disorder. Biological Psychiatry, 39, 839–844.
Claridge, G. S. (1987). The schizophrenias as nervous types revisited. British Journal of Psychiatry, 151, 735–743.
Coryell, W. (1996). Psychotic depression. Journal of Clinical Psychiatry, 57(suppl. 3), 27–31.
Delespaul, P., deVries, M., & van Os, J. (2002). Determinants of occurrence and recovery from hallucinations in daily life. Social Psychiatry and Psychiatric Epidemiology, 37, 97–104.
Dierks, T., Linden, D. E. J., Jandi, M., Formisano, E., Goebel, R., Lanfermann, H., et al. (1999). Activation of Heschl’s gyrus during auditory hallucinations. Neurone, 22, 615–621.
Esquirol, J. E. D. (1832). Sur les illusions des sens chez alienes. Archives Generales de Medicine, 2, 5–23.
Famularo, R., Kinscherff, R., & Fenton, T. (1992). Psychiatric diagnoses of maltreated children: Preliminary findings. Journal of the American Academy of Child and Adolescent Psychiatry, 31, 863–867.
Feiring, C., Taska, L., & Lewis, M. (2002). Adjustment following sexual abuse discovery: The role of shame and attributional style. Developmental Psychology, 38, 79–92.
Ford, J. M., & Mathalon, D. H. (2004). Electrophysiological evidence of corollary discharge dysfunction in schizophrenia during talking and thinking. Journal of Psychiatric Research, 38, 37–46.
Frith, C. D. (1992). The cognitive neuropsychology of schizophrenia. Hillsdale, NJ: Lawrence Erlbaum.
Gallagher, A. G., Dinin, T. G., & Baker, L. V. J. (1994). The effects of varying auditory input on schizophrenic hallucinations: A replication. British Journal of Medical Psychology, 67, 67–76.
Garcia-Montes, J. M., Cangras, A., Perez-Alvarez, M., Fidalgo, A. M., & Gutierrez, O. (2006). The role of meta-cognitions and thought control techniques in predisposition to auditory and visual hallucinations. British Journal of Clinical Psychology, 45, 309–317.
Garrett, M., & Silva, R. (2003). Auditory hallucinations, source monitoring, and the belief that “voices” are real. Schizophrenia Bulletin, 29, 445–457.
Goodman, L. A., Rosenberg, S. D., Mueser, K., & Drake, R. E. (1997). Physical and sexual assault history in women with serious mental illness: Prevalence, correlates, treatment, and future research directions. Schizophrenia Bulletin, 23, 685–696.
Goodwin, F. K., & Jamison, K. R. (1990). Manic-depressive illness. Oxford: Oxford University Press.
Gould, L. N. (1948). Verbal hallucinations and activity of vocal musculature. American Journal of Psychiatry, 105, 367–372.
Gould, L. N. (1949). Auditory hallucinations and subvocal speech. Journal of Nervous and Mental Disease, 109, 418–427.
Gould, L. N. (1950). Verbal hallucinations and automatic speech. American Journal of Psychiatry, 107, 110–119.
Green, P., & Preston, M. (1981). Reinforcement of vocal correlates of auditory hallucinations by auditory feedback: A case study. British Journal of Psychiatry, 139, 204–208.
Grimby, A. (1998). Hallucinations following the loss of a spouse: Common and normal events among the elderly. Journal of Clinical Geropsychology, 4, 65–74.
Haddock, G., Slade, P. D., & Bentall, R. P. (1995). Auditory hallucinations and the verbal transformation effect: The role of suggestions. Personality and Individual Differences, 19, 301–306.
Hammersley, P., Dias, A., Todd, G., Bowen-Jones, K., Reilly, B., & Bentall, R. P. (2003). Childhood trauma and hallucinations in bipolar affective disorder: A preliminary investigation. British Journal of Psychiatry, 182, 543–547.
Hamner, M. B., Fueh, B. C., Ulmer, H. G., Huber, M., Twomey, T. J., Tyson, C., et al. (2000). Psychotic features in chronic posttraumatic stress disorder and schizophrenia: Comparative severity. Journal of Nervous and Mental Disease, 188, 217–221.
Hardy, A., Fowler, D., Freeman, D., Smith, B., Steel, C., Evans, J., et al. (2005). Trauma and hallucinatory experiences in psychosis. Journal of Nervous and Mental Disease, 193, 501–507.
Hoffman, R. E. (1986). Verbal hallucinations and language production processes in schizophrenia. Behavioral and Brain Sciences, 9, 503–548.
Honig, A., Romme, M. A. J., Ensink, B. J., Escher, S. D. M. A. C., Pennings, M. H. A., & DeVries, M. W. (1998). Auditory hallucinations: A comparison between patients and nonpatients. Journal of Nervous and Mental Disease, 186, 646–651.
Inouye, T., & Shimizu, A. (1970). The electromyographic study of verbal hallucination. Journal of Nervous and Mental Disease, 151, 415–422.
Jablensky, A. (1995). Schizophrenia: The epidemiological horizon. In S. R. Hirsch & D. R. Weinberger (Eds.), Schizophrenia (pp. 206–252). Oxford: Blackwell.
Jaspers, K. (1913/1963). General psychopathology (Hoenig, J., & Hamilton, M. W., Trans.). Manchester: Manchester University Press.
Johns, L. C., Gregg, L., Allen, P., & McGuire, P. K. (2006). Impaired verbal self-monitoring in psychosis: effects of state, trait and diagnosis. Psychological Medicine, 36, 465–474.
Johns, L. C., Gregg, L., Allen, P., Vythelingum, G. N., & McGuire, P. K. (2006). Verbal self-monitoring and auditory verbal hallucinations in psychosis: Symptom or syndrome specific? Psychological Medicine, 36, 465–474.
Johns, L. C., & McGuire, P. K. (1999). Verbal self-monitoring and auditory hallucinations in schizophrenia. Lancet, 353, 469–470.
Johns, L. C., Rossell, S., Frith, C., Ahmad, F., Hemsley, D., Kuipers, E., et al. (2001). Verbal self-monitoring and auditory hallucinations in people with schizophrenia. Psychological Medicine, 31, 705–715.
Johnson, M. K., Hashtroudi, S., & Lindsay, D. S. (1993). Source monitoring. Psychological Bulletin, 114(1), 3–28.
Jones, S. R., & Fernyhough, C. (2007). Neural correlates of inner speech and auditory verbal hallucinations: A critical review and theoretical integration. Clinical Psychology Review, 27, 140–154.
Juninger, J., & McGuire, L. (2001). The paradox of command hallucinations. Psychiatric Services, 52, 385.
Kasper, M. E., Rogers, R., & Adams, P. A. (1996). Dangerousness and command hallucinations: An investigation of psychotic inpatients. Bulletin of the American Academy of Psychiatry and the Law, 24, 219–224.
Keefe, R. S. E. (2002). Source-monitoring deficits for self-generated stimuli in schizophrenia: Multinomial modelling of data from three sources. Schizophrenia Research, 57, 51–67.
Keefe, R. S. E., Arnold, M. C., Bayen, U. J., & Harvey, P. D. (1999). Source monitoring deficits in patients with schizophrenia: A multinomial modelling analysis. Psychological Medicine, 29, 903–914.
Keefe, R. S. E., Krabbendam, L., Dautzenberg, J., Jolles, J., & Merckelbach, H. (2005). Confusing thoughts and speech: Source monitoring and psychosis. Psychiatry Research, 133, 57–63.
Kohlberg, L., Yaeger, J., & Hjertholm, E. (1968). Private speech: Four studies and a review of theories. Child Development, 39, 691–736.
Laroi, F., van der Linden, M., & Marczewski, P. (2004). The effects of emotional salience, cognitive effort, and meta-cognitive beliefs on a reality monitoring task in hallucination-prone subjects. British Journal of Clinical Psychology, 43, 221–233.
Laroi, F., & Woodward, T. S. (2007). Hallucinations from a cognitive perspective. Harvard Review of Psychiatry, 15, 109–117.
Latasker, T., van Os, J., Drukker, M., Henquet, C., Feron, F., Gunther, N., et al. (2006). Childhood victimisation and developmental expression of non-clinical delusional ideation and hallucinatory experiences: Victimisation and non-clinical psychotic experiences. Social Psychiatry and Psychiatric Epidemiology, 41, 423–428.
Lattuada, E., Serretti, A., Cusin, C., Gasperini, M., & Smeraldi, E. (1999). Symptomatologic analysis of psychotic and non-psychotic depression. Journal of Affective Disorders, 54, 183–187.
Leudar, I., Thomas, P., McNally, D., & Glinsky, A. (1997). What voices can do with words: Pragmatics of verbal hallucinations. Psychological Medicine, 27, 885–898.
Lobban, F., Haddock, G., Kinderman, P., & Wells, A. (2002). The role of metacognitive beliefs in auditory hallucinations. Personality and Individual Differences, 32, 1351–1363.
Margo, A., Hemsley, D. R., & Slade, P. D. (1981). The effects of varying auditory input on schizophrenic hallucinations. British Journal of Psychiatry, 139, 122–127.
McGrath, J. J. (2005). Myths and plain truths about schizophrenia epidemiology. Acta Psychiatrica Scandinavica, 111, 4–11.
McGuigan, F. J. (1966). Covert oral behavior and auditory hallucinations. Psychophysiology, 3, 73–80.
McGuigan, F. J. (1978). Cognitive psychophysiology: Principles of covert behavior. Englewood Cliffs, NJ: Prentice Hall.
McGuire, P. K., Shah, G. M. S., & Murray, R. M. (1993). Increased blood flow in Broca’s area during auditory hallucinations. Lancet, 342, 703–706.
McNeil, D. E., Eisner, J. P., & Binder, R. L. (2000). The relationship between command hallucinations and violence. Psychiatric Services, 51, 1288–1292.
Morrison, A. P. (2001). The interpretation of intrusions in psychosis: An integrative cognitive approach to hallucinations and delusions. Behavioural and Cognitive Psychotherapy, 29, 257–276.
Morrison, A. P., & Baker, C. A. (2000). Intrusive thoughts and auditory hallucinations: A comparative study of intrusions in psychosis. Behaviour Research and Therapy, 38, 1097–1106.
Morrison, A. P., Frame, L., & Larkin, W. (2003). Relationships between trauma and psychosis: A review and integration. British Journal of Clinical Psychology, 42, 331–353.
Morrison, A. P., & Petersen, T. (2003). Trauma, metacognition and predisposition to hallucinations in non-patients. Behavioural and Cognitive Psychotherapy, 31, 235–246.
Morrison, A. P., & Wells, A. (2003). Metacognition across disorders: A comparison of patients with hallucinations, delusions, and panic disorder with non-patients. Behaviour Research and Therapy, 41, 251–256.
Mueser, K. T., & Butler, R. W. (1987). Auditory hallucinations in combat-related chronic posttraumatic stress disorder. American Journal of Psychiatry, 144, 299–302.
Mueser, K. T., Goodman, L. B., Trumbetta, S. L., Rosenberg, S. D., Osher, F. C., Vidaver, R., et al. (1998). Trauma and posttraumatic stress disorder in severe mental illness. Journal of Consulting and Clinical Psychology, 66, 493–499.
van Os, J., Hanssen, M., Bijl, R. V., & Ravelli, A. (2000). Strauss (1969) revisited: A psychosis continuum in the normal population? Schizophrenia Research, 45, 11–20.
Nayani, T. H., & David, A. S. (1996). The auditory hallucination: A phenomenological survey. Psychological Medicine, 26, 179–192.
Neria, Y., Bromet, E. J., Sievers, S., Lavelle, J., & Fochtmann, L. J. (2002). Trauma exposure and posttraumatic stress disorder in psychosis: Findings from a first-admission cohort. Journal of Consulting and Clinical Psychology, 70, 246–251.
Paolucci, E. O., Genuis, M. L., & Violato, C. (2001). A meta-analysis of the published research on the effects of child sexual abuse. Journal of Psychology, 135, 17–36.
Piaget, J. (1926). The language and thought of the child. London: Routledge & Kegan Paul.
Putnam, F. W. (2003). Ten-year update review: Child sexual abuse. Journal of the American Academy of Child and Adolescent Psychiatry, 42, 269–278.
Rankin, P., & O’Carrol, P. (1995). Reality monitoring and signal detection in individuals prone to hallucinations. British Journal of Clinical Psychology, 34, 517–528.
Read, J., Agar, K., Argyle, N., & Aderhold, V. (2003). Sexual and physical abuse during childhood and adulthood as predictors of hallucinations, delusions and thought disorder. Psychology and Psychotherapy: Theory, Research, and Practice, 76, 1–22.
Read, J., & Argyle, N. (1999). Hallucinations, delusions, and thought disorder among psychiatric patients with a history of child abuse. Psychiatric Services, 50, 1467–1472.
Read, J., van Os, J., Morrison, A. P., & Ross, C. A. (2005). Childhood trauma, psychosis and schizophrenia: A literature review and clinical implications. Acta Psychiatrica Scandinavica, 112, 330–350.
Romme, M., & Escher, A. (1989). Hearing voices. Schizophrenia Bulletin, 15, 209–216.
Sanders, B., & Giolas, M. H. (1991). Dissociation and childhood trauma in psychologically disturbed adolescents. American Journal of Psychiatry, 148, 50–54.
Sansonnet-Hayden, H., Haley, G., Marriage, K., & Fine, S. (1987). Sexual abuse and psychopathology in hospitalized adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 26, 753–757.
Sartorius, N., Jablensky, A., Korten, A., Ernberg, G., Anker, M., Cooper, J. E., et al. (1986). Early manifestations and first contact incidence of schizophrenia in different cultures. Psychological Medicine, 16, 909–928.
Schneider, K. (1959). Clinical psychopathology. New York: Grune & Stratton.
Shergill, S. S., Bullmore, E., Simmons, A., Murray, R. M., & McGuire, P. K. (2000). Functional neuroanatomy of auditory verbal imagery in schizophrenia patients with hallucinations. American Journal of Psychiatry, 157, 1691–1693.
Shevlin, M., Dorahy, M., & Adamson, G. (2007). Childhood traumas and hallucinations: An analysis of the National Comorbidity Survey. Journal of Psychiatric Research, 41, 222–228.
Sidgewick, H. A. (1894). Report of the census on hallucinations. Proceedings of the Society for Psychical Research, 26, 259–394.
Siegel, R. K. (1984). Hostage hallucinations: Visual imagery induced by isolation and life-threatening stress. Journal of Nervous and Mental Disease, 172, 264–272.
Silbersweig, D. A., Stern, E., Frith, C., Cahill, C., Holmes, A., Grootoonk, S., et al. (1995). A functional neuroanatomy of hallucinations in schizophrenia. Nature, 378, 176–179.
Slade, P. D., & Bentall, R. P. (1988). Sensory deception: A scientific analysis of hallucination. London: Croom-Helm.
Sritharan, A., Line, P., Sergejew, A., Silberstein, R., Egan, G., & Copolov, D. (2005). EEG coherence measures during auditory hallucinations in schizophrenia. Psychiatry Research, 136, 189–200.
Startup, M. (1999). Schizotypy, dissociative experiences, and childhood abuse: Relationships among self-report measures. British Journal of Clinical Psychology, 38, 333–344.
Stephane, M. (2003). The internal structure and phenomenology of auditory verbal hallucinations. Schizophrenia Research, 61, 185–193.
Tarrier, N., Lewis, S., Haddock, G., Bentall, R. P., Drake, R., Dunn, G., et al. (2004). 18-month follow-up of a randomized controlled trial of cognitive-behaviour therapy in first episode and early schizophrenia. British Journal of Psychiatry, 184, 231–239.
Tien, A. Y. (1991). Distribution of hallucinations in the population. Social Psychiatry and Psychiatric Epidemiology, 26, 287–292.
Varese, F., Barkus, E., & Bentall, R. P. (2011a). Dissociation mediates the relationship between childhood trauma and hallucination-proneness. Psychological Medicine, 42, 1025–1036.
Varese, F., Barkus, E., & Bentall, R. P. (2011b). Dissociative and metacognitive factors in hallucination-proneness when controlling for comorbid symptoms. Cognitive Neuropsychiatry, 16, 193–217.
Varese, F., Smeets, F., Drukker, M., Lieverse, R., Lataster, T., Viechtbauer, W., et al. (2012). Childhood adversities increase the risk of psychosis: A meta-analysis of patient-control, prospective, and cross-sectional cohort studies. Schizophrenia Bulletin, 38, 661–671.
Vercammen, A., de Haan, E. H. F., & Aleman, A. (2008). Hearing a voice in the noise: Auditory hallucinations and speech perception. Psychological Medicine, 38, 1177–1184.
Versmissen, D., Janssen, I., Johns, L. C., McGuire, P. K., Drucker, M., & Campo, J. A. (2007). Verbal self-monitoring in psychosis: a non-replication. Psychological Medicine, 37, 569–576.
Vygotsky, L. S. V. (1962). Thought and language. Cambridge, MA: MIT Press.
Walach, H., Buchheld, N., Buttenmuller, V., Kleinknecht, N., & Schmidt, S. (2006). Measuring mindfulness: The Freiburg Mindfulness Inventory (FMI). Personality and Individual Differences, 40, 1543–1555.
West, D. J. (1948). A mass observation questionnaire on hallucinations. Journal of the Society for Psychical Research, 34, 187–196.
Whitfield, C. L., Dube, S. R., Felitti, V. J., & Anda, R. F. (2005). Adverse childhood experiences and hallucinations. Child Abuse and Neglect, 29, 797–810.
Wiffen, V., & MacIntosh, H. B. (2005). Mediators of the link between childhood sexual abuse and emotional distress. Trauma, Violence, and Abuse, 6, 24–39.