Case
A 35 year old obese woman presents with headache, pulse synchronous tinnitus, and blurred vision. Visual acuity is 20/20 (6/6) OU. The pupil exam was normal OU. Automated perimetry showed a superior and inferior nerve fiber layer defect OU with a mean deviation of −27 decibels (dB) OD and −14 dB OS. Fundus exam showed Frisen grade 3 papilledema OU. Cranial magnetic resonance imaging (MRI) with and without gadolinium was negative for intracranial lesion and MR venography was negative for venous sinus stenosis or thrombosis. Lumbar puncture showed a markedly elevated opening pressure of 40 cm of water but normal cerebrospinal fluid contents.
Introduction
Idiopathic intracranial hypertension (IIH) is a neurologic disorder of increased CSF pressure of unknown cause, first described in the literature in the late nineteenth century as reversible elevation of intracranial pressure (ICP). In 1955, Foley introduced the term “benign intracranial hypertension”. The term “benign” no longer accurately describes the disease given the propensity for visual loss [1].
Commonly, it presents with the symptoms of increased intracranial pressure (ICP) in an overweight female of reproductive age, although it can occur in in men, and patients of all weights and ages. Per the modified Dandy Criteria, diagnosis is made when a patient (1) is awake and alert; (2) presents with signs and symptoms consistent with increased ICP; (3) lacks focal neurological signs; (4) neuroimaging is negative for any deformity, displacement or obstruction of the ventricular system; (5) cerebrospinal fluid (CSF) opening pressure is found to be greater than 200–250 mm of water; (6) no other explanation of increased ICP is found [2–7]. Today, the diagnosis of IIH is still maintained in the presence of unilateral or bilateral abducens palsy, and in the presence of radiological evidence of distal transverse sinus narrowing, although this may be primary or secondary [8].
The reported incidence of IIH varies from 1 to 3 per 100,000 in the general population with peak incidence occurring during the third decade of life [9]. IIH disproportionately affects obese women of child-bearing age. According to a population study done in Minnesota, the incidence was 22 per 100,000 in obese women aged 15–44, compared with 6.8 per 100,000 among all women in the same age group. The general incidence averaged 1.8 per 100,000. According to the same study, the incident of IIH has increased from 1 per 100,000 in the years 1990–2001 to 2.4 per 100,000 in 2002–2014 [10]. The increasing incidence of IIH is attributed to the increasing rate of obesity, it being present in 90% of those with IIH. Increased BMI and weight gain velocity is associated with increased risk of developing IIH and a worse prognosis. Those with BMI >40 have greater incidence of vision loss [11]. Not only is obesity a risk factor, but weight gain in the non-obese also increases risk. A study found that even weight gain of 5–15% can increase the risk of IIH [12].
In the pediatric population, the incidence is 0.71 per 100,000 in children 1–16 years old. The incidence, female predominance and association with obesity all increase with age. In ages 1–7, female to male ratio was 1:1, whereas in ages 8–16, the ratio increased to 2:1. This suggests that after the age of 7, being female and obese increases risk of IIH, similar to the adult population [13].
Despite the large body of scientific literature on IIH, the pathophysiology of IIH remains uncertain. It is most likely caused by a combination of potentially interactive factors including increased CSF production, reduced CSF absorption, increased cerebral venous pressure, venous sinus stenosis and increased brain water content. The majority of CSF is produced by the choroid plexus via ion transporters which govern the movement of water and ions. A dysregulation in these channels may play a role in IIH. Aquaporin 1 is one of these transport channels that is down regulated by acetazolamide, a drug commonly used to treat IIH [14]. Additionally, a study of CSF clearance using radioisotopic cisternography in patients with IIH found increased arachnoid resistance to clearance of CSF [15]. Although the relationship between obesity and IIH has been well established, the mechanism linking the two is still undetermined [16, 17]. An early theory attributed increased ICP from the increased venous pressure caused by the abdominal mass in obesity. However, this theory does not explain the much higher prevalence of obesity compared to IIH. More recently, with the recognition that adipose tissue is an endocrine organ and moreover, that it secretes, proteins, including cytokines, chemokines and homones, it has been demonstrated that activated adipose recruits macrophages that recruit inflammatory mediators, the most relevant being adipokine, leptin, and other hormones, which may play an important role in the generation of IIH in the presence of obesity [18–20].
Symptoms and Signs
Frequency of symptoms in patients with IIH
Symptom | Occurrence (% of patients) |
|---|---|
Headache | 76–94% |
Transient visual obscurations | 68–72% |
Pulsatile tinnitus | 52–60% |
Back pain | 53% |
Dizziness | 52% |
Neck pain | 42% |
Visual loss or blurring | 32% |
Cognitive disturbances | 20% |
Radicular pain | 19% |
Horizontal diplopia | 18% |
The sign most often found on physical exam in IIH is bilateral papilledema caused by the transmission of CSF through the optic nerve sheath. Papilledema ultimately causes visual loss as axoplasmic transport in the optic nerve is interrupted due to the increased pressure. However, severity of papilledema is a poor predictor for the degree of vision loss. The most common visual field defects found in IIH are an enlarged blind spot and partial arcuate defect worse in the inferior field. While permanent, global visual loss is rare, it is the most serious morbidity of this disease and the risk is high if the disease is untreated and there is persistent, chronic papilledema [24–27].
Once papilledema or abducens palsies are found on exam, ancillary tests are helpful in confirming the diagnosis and monitoring progression of the disease. Visual field of the central 24–30 degrees can be tested with standard automated perimetry testing. The earliest visual defect in papilledema is the loss of the inferonasal portion of the visual field with progressive loss of peripheral fields following [24, 25, 27]. Visual acuity is usually spared until late manifestations of the disease. OCT is also helpful in monitoring optic neuropathy progression as well as response to treatment. OCT quantifies papilledema by measuring optic disc volume, total retinal thickness and retinal nerve fiber layer (RNFL) thickness , which is higher in patients with papilledema and also macular thickness, which may diminish progressively with sustained papilledema [26, 28, 29]. Notwithstanding the fact that maintained papilloedema is associated with visual loss over time, currently it is unclear if RNFL thickness is directly correlated with visual dysfunction [28, 30].
A noninvasive, rapid and high accuracy diagnostic test for determining increased ICP is ocular ultrasound, which can measure the optic nerve sheath diameter. The 30° test is employed on ultrasound to detect papilledema. An increase in diameter of the optic nerve in primary gaze and a 25% decrease in eccentric gaze signifies increased subarachnoid fluid surrounding the optic nerve [31].
Diagnosis
- 1.
Signs and symptoms of increased intracranial pressure including papilledema, headaches, nausea and vomiting
- 2.
Alert and awake
- 3.
No focal neurological findings except abducens nerve palsy
- 4.
Negative findings on neuroimaging other than evidence of increased CSF pressure (specifically, absence of deformities, displacement or obstruction of ventricular system)
- 5.
Elevated lumbar puncture opening pressure > 250 mmH2O
- 6.
No other causes of increased ICP found
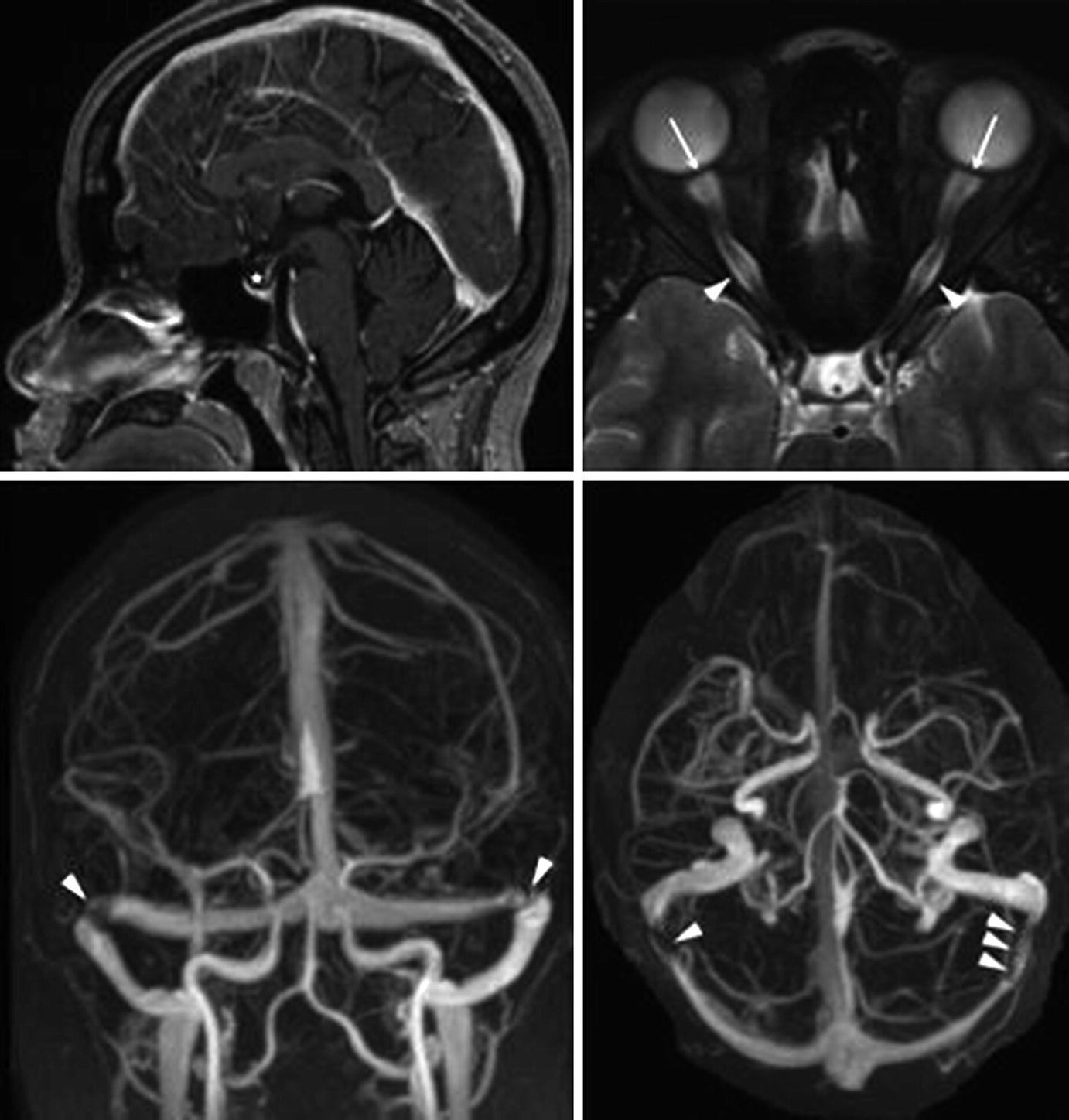
Magnetic resonance imaging findings in idiopathic intracranial hypertension (IIH). (Top left) Empty sella (star) on sagittal T1-weighted imaging. (Top right) Dilated optic nerve sheaths (arrowheads) and posterior globe flattening (arrowheads) on axial T2-weighted imaging. (Bottom left and right) Bilateral transverse cerebral venous stenosis (arrowheads), as seen on contrast-enhanced magnetic resonance venography. Source: Bruce, Beau B, Valerie Biousse, and Nancy J Newman. “Update on idiopathic intracranial hypertension.” American Journal of Ophthalmology 152.2 (2011):163–169
Lumbar puncture must be performed to obtain the opening pressure as well as to analyze the CSF. The LP should be performed with the patient relaxed and in the lateral decubitus position to avoid artificial changes in pressure readings. As opening pressure readings are often unreliable, a normal opening pressure should not exclude IIH as the diagnosis. Readings should always be correlated with the history and physical examination. Additionally, even though the cutoff for elevated opening pressure is 250 mm H2O, it must be noted that this represents the 95th percentile or normal and that 2% of adults can have an opening pressure > 25 mm H2O normally. The LP can be diagnostic as well as therapeutic and patients should be asked about improvements in symptoms a few days post LP. A normal opening pressure does not exclude the diagnosis however as CSF pressure is dynamic and may change throughout the day [33]. The CSF should also be collected and analyzed for cell count, cytology, culture, and measurement of glucose, protein, and electrolyte concentrations to exclude other causes for increased ICP.
Management
Once IIH is diagnosed, secondary causes should be investigated, such as obstructive sleep apnea, hormonal abnormalities or certain medications. These root causes should be addressed by eliminating the offending agent or treating the underlying disease. Management of IIH is multifactorial and aims to achieve the goal of preventing vision loss and diminishing symptoms. There are variable courses of the disease, with some patients going into remission with a lumbar puncture, to others with rapidly declining vision over days despite lumbar puncture. When vision loss is not present or mild, conservative measures with risk reduction and medical management are used. However, in moderate to severe vision loss, worsening of vision loss, or symptoms refractory to medical management, surgical interventions should be considered. As visual decline can occur rapidly, a plan for emergent surgery should be in place.
Conservative Management
Weight loss is a well-established means of treating IIH. Several studies have correlated a lower BMI with better outcomes and have shown that weight reduction of 5–10% can reduce papilledema, ICP and improve symptoms [34–38]. As weight loss via diet and exercise is difficult to maintain, bariatric surgery is an effective option for longer term weight loss. It has been reported to lead to resolution of papilledema and improvement of headaches. Although post-surgery, vitamins must be supplemented to prevent nutrition related ophthalmologic complications [39–41].
Acetazolamide has been the mainstay of treatment of IIH. The carbonic anhydrase inhibitor decreases CSF production and thus, lowers ICP. The Idiopathic Intracranial Hypertension Treatment Trial (2014) [42] showed that for patients with mild vision loss, acetazolamide up to 4 g per day in conjunction with weight loss was an effective treatment. It demonstrated improvements in papilledema, intracranial pressure, and quality of life. Furosemide and topiramate, both weak carbonic anhydrase inhibitors, have also been used to treat IIH in conjunction with acetazolamide or alone in patients who cannot tolerate acetazolamide. Neither has been shown to be as effective as acetazolamide [43–45]. High dose steroids can be used as a bridge to surgery for those with severe or rapidly progressive vision loss. Though effective, due to its side effects in prolonged use, it is not prescribed for long term management.
Surgical Management
- 1.
Failure to prevent progressive visual loss.
- 2.
Failure to control headache or other symptoms.
- 3.
Failure of the physician to adequately communicate to the patient the nature of the disease and the likelihood of spontaneous improvement with good conservative management.
- 4.
Failure of the patient to accept and enact an adequate management regime that often involves a degree of self-deprivation and exercise.
Clearly however, there is a place for surgical intervention.
For visual loss, optic nerve sheath fenestration (ONSF) is the treatment of choice and involves making a series of cuts in the retrobulbar optic nerve sheath to reduce CSF pressure on the optic nerve and prevent further nerve damage. ONSF is used for those with moderate to severe or progressive visual loss, but is not as effective at treating headaches as there is minimal effect on ICP. It leads to rapid reduction of papilledema on the operated side and also improves the contralateral side [46, 47]. In a small percentage of patients, vision loss may worsen and another ONSF or a CSF shunt may be required. Thus, serial monitoring of vision is necessary following operation.
Alternatively, CSF diversion can be achieved via several types of shunt including lumbar-peritoneal (LPS) and ventriculo-peritoneal (VPS). Shunting of CSF leads to rapid decrease in ICP and improves related symptoms. It is preferred in patients with severe headaches refractory to medical management. However, most shunts will require revision in the long term due to failure. While VPS have a higher patency rate and are more effective at decreasing ICP, it is technically more challenging to place due to decreased ventricular size in IIH patients [48, 49]. Complications include low pressure headaches, cerebellar tonsillar descent and infections.
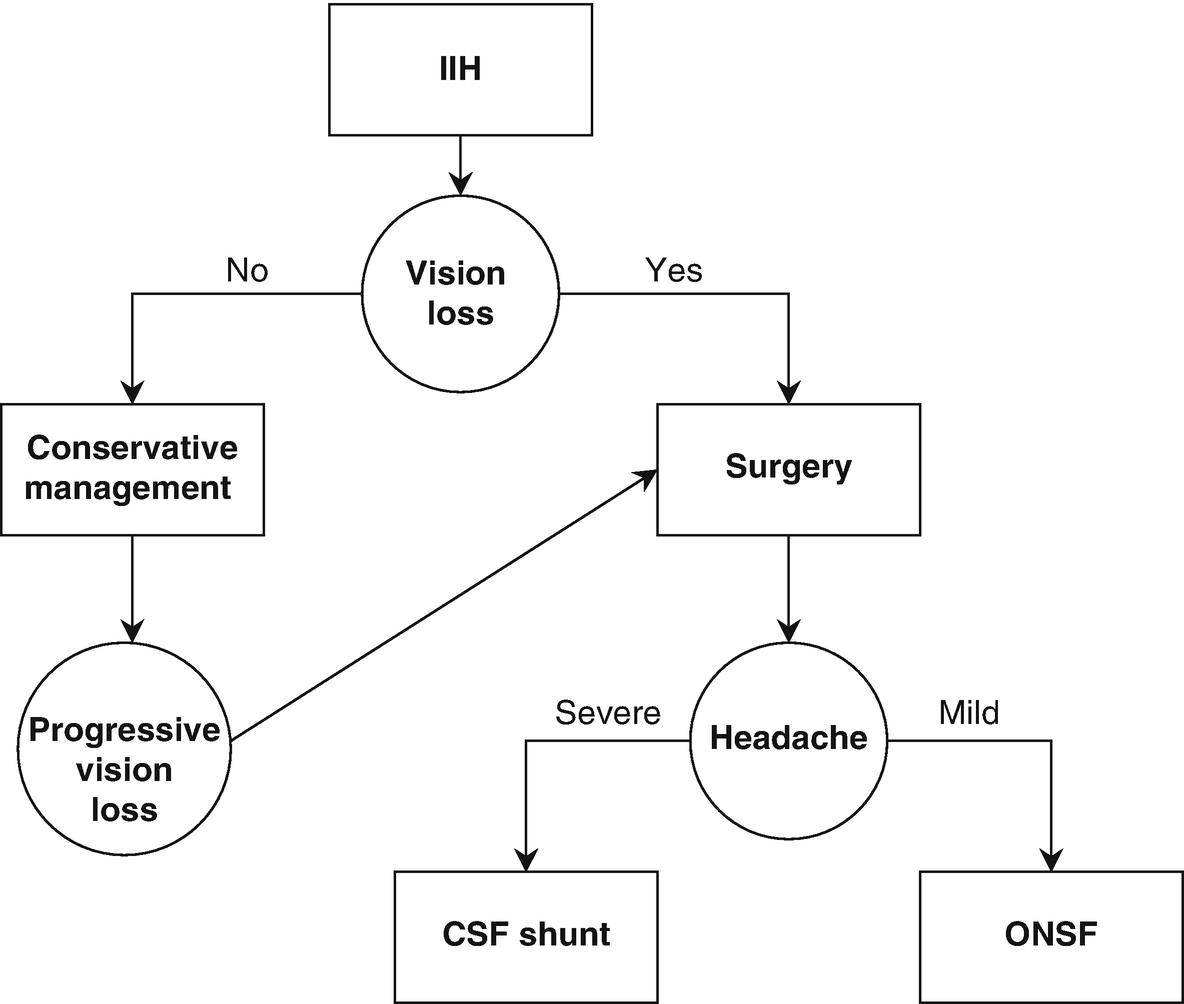
Treatment algorithm for IIH
Sheath Fenestration Versus Shunt Placement
Optic Nerve Sheath Fenestration
DeWecker reported the first optic nerve sheath fenestration in 1872 by incising the meninges surrounding the retro-orbital optic nerve in cases of neuroretinits [53]. It was first used as a treatment for pressure on the optic nerve in the late nineteenth century (for a review see Sergott [47]). Subsequently, it was used to treat papilledema in 1964 by Hayreh who opened the optic nerve sheath and found resolution of optic disc swelling. Thus, he established that papilledema is caused by the mechanical transfer of pressure from increased CSF via the optic nerve sheath [54]. Since then, it has been well established as a treatment option for vision loss due to papilledema in IIH and preventing further optic nerve damage. Complete resolution of papilledema can occur as quickly as 2 weeks, with dramatic improvement occurring within days after ONSF [47, 55]. In meta-analyses of visual outcomes following ONSF, visual acuity remained stable or improved in 90% of patients. Two studies quantified visual field improvement by Humphrey VF mean deviation (MD). The mean preoperatively was −15.5 dB and improved to a mean postoperative MD of −9.1 dB. Papilledema as measured by fundoscopy remained stable long-term in 10% and improved in 90% of patients [4, 56].
Improvement in vision post-ONSF depends on several factors including pre-operative visual acuity, acuity of symptoms, age, and shorter duration of surgery. Case series have shown that patients with only mild visual field defects were more likely to have improved visual acuity (VA) and visual field (VF) postoperatively, whereas patients with more severe visual field defects have stabilized VA and VF postoperatively. Patients with acute symptoms had better responses after ONSF than patients with chronic, atrophic disc edema. Additionally, more favorable results are seen in younger patients [55, 57, 58]. It has also been demonstrated that unilateral ONSF can reduce papilledema in the ipsilateral and contralateral eye. The 62 patient study by Alsuhaibani et al. demonstrated that bilateral ONSF may not be necessary to treat papilledema in IIH. Although the reduction in papilledema was the greatest on the operated eye, at 12 month follow up [46].
In tracking long term improvement, the 2000 Banta and Farris study showed that 90% of patients with improved vision post-operatively maintained this improvement at 6 month follow up [59]. The study was one of the largest retrospective, non-comparative, interventional case series studying the effectiveness of ONSF. It included 158 eyes and found that 88% of patients had improved or stable VF and 97% had stable or improved VA post-operatively. In the 10% of patients who experienced visual decline despite initial success in ONSF, the timeline to decline was variable and spanned a 5 year period post-operation. In another study of 75 eyes by Spoor et al., visual decline after successful surgery was reported to be 32%. These patients subsequently underwent a repeat ONSF with 75% maintaining stabilized or improved vision up to 36 month follow up. Thus, despite initial success, visual field monitoring is paramount and repeat ONSF may be required [58].
ONSF can also be performed on those who have failed to see visual improvement on LPS placement. Sergott et al. demonstrated that ONSF restored visual acuity and visual field loss in six patients who failed to regain visual function after one or more lumbar-peritoneal shunts. The length of time between LPS failure and ONSF did not affect vision improvement post operatively. Since LPS often require several revisions, ONSF may be a more effective and efficient way of maintaining restored vision [47].
Optic nerve sheath fenestration has also been shown to be as effective in children with IIH as with an adult population. Studies have shown that the procedure is not only safe in the pediatric population, but can result in improved visual acuity, mean color vision test performance, and mean optic nerve appearance [60, 61]. ONSF may also result in reduction in headache but is not widely accepted as appropriate therapy for headache in the absence of visual impairment [47, 62, 63].
Complications
As with any surgical procedure there are known complications to ONSF. The most commonly cited ones being diplopia, anisocoria, hemorrhage, ocular dysmotility, retinal artery occlusion, iris sphincter paresis, and sudden IOP increase. Despite the range of possible complications, the majority are transient and self-limiting. There is not a clear consensus on the rate of complications in the literature, with a range of 5–45% being reported. In a 2017 meta-analysis by Kalyvas et al. including 15 studies, VF worsened in 8% and VA in 11% post-operatively. Complication rate averaged 26% in patients with transient diplopia being the most common [64].
The complications of ONSF differ by surgery method. Medial transconjunctival access is thought to be one of the safest and most accepted methods. It allows access to the optic nerve without removal of the orbital wall. In the study by Chandrasekaran et al., in which all patients underwent surgery with this approach, the complication rate was 15.6% out of a total of 52 patients. The complications included anisocoria, diplopia and optic disc hemorrhage, all of which were self- limiting and resolved without intervention [57]. Pelton et al. studied OSNF with a superomedial lid crease approach and the complication rate was 22%. Complications included tonic pupil, transient vertical diplopia, and transient medial ptosis [65]. Plotnik and Kosmorsky reported one of the highest complication rates 40% using a medial orbitotomy with complications being temporary motility disorders, pupillary dysfunction, central retinal artery occlusions, transient outer retinal ischemia, and branch retinal artery occlusion [66]. Moreau et al. studied a large number of patients with undefined orbital approaches. The complication rate was 7.2%, with complications including ocular dysmotility and corneal dellen [67], a significantly better outcome than other studies. Corbett et al. performed ONSFs on 40 eyes using the lateral orbitotomy approach. Significant complications included permanent tonic pupils, retrobulbar hemorrhage, and sixth nerve palsy [63]. Endoscopic optic nerve decompression is recently developed approach to OSNF. Transnasal endoscopic surgery offers minimally invasive access to the optic nerve, while avoiding the medial rectus disinsertion required for the transconjunctival approach. No major surgical complications were reported in the 34 patients in whom EOND was performed. However, risks related to endoscopic include CSF leak, meningitis, epistaxis, subcutaneous orbital emphysema, and visual deterioration secondary to thermal injury from drilling the optic canal, and CNS infection [68].
In summary, ONSF has been a successful procedure to prevent decline and improve vision in IIH, but there are complications including direct orbital and intraocular related effects and a worsening of visual function. While the procedure is generally safe, systemic anesthesia-related complications can also obviously occur. There are several surgical approaches to this surgery and each has its unique complications. The decision of which is approach to use is dependent on surgeon preference as there is no clear optimum approach. It has been demonstrated that unilateral ONSF is adequate in achieving bilateral resolution, thus making bilateral surgery unnecessary.
CSF Shunts
CSF shunting procedures , including LPS, VPS, and ventriculo-pleural, is another procedure frequently utilized to decrease CSF and thus ICP in IIH. Shunts have proven to be highly effective in resolving headaches and are preferred in patients with refractory headaches or headaches failing maximal medical therapy alone. Reports have shown that immediate resolution of headaches post-surgery range between 82 and 100% [48]. Long term success is dependent on duration of symptoms prior to the surgery and whether or not there is shunt failure. Preoperative presence of papilledema and headache for less than 2 years is associated with better outcomes than those who’d had longer duration of IIH. In these patients, up to 90% remained headache free 2 years post-surgery. In those who experienced shunt failure, revisions led to continued resolution of the headaches in the long term for the majority of patients [48]. However, in a fraction, headaches remained despite shunt revision. A meta-analysis of LPS for IIH treatment showed that visual acuity was stable or improved in 89% of patients with mean Snellen of 6/18 and 6/12 pre and post-operatively. Visual field remained stable or improved in 100% of patients [56].
LPS Versus VPS
A LPS involves placing a catheter to connect the subarachnoid space between two vertebrae with the peritoneal cavity. In doing so, it creates an anastomoses for CSF to travel from the subarachnoid space to the peritoneum and hence decrease CSF volume and pressure. A VPS on the other hand connects the cerebral ventricles with the peritoneum. Due to the difficulty of placing a shunt in an non-dilated ventricle, LPS has been the mainstay for CSF shunting in IIH in the past. However, with the increasing use of stereotaxic guided placement and lower revision rates, VPS are becoming more accessible and preferred. Studies have shown that visual symptoms, papilledema and headaches improve equally in both LPS and VPS. However, they differ in the associated complications. While LPS has the benefit of limiting intracranial complications, it often requires revision more often and sooner. In one report, the average time between shunt insertion and shunt replacement in LPS averages 9 months, although often, the shunts last less than 6 months. 2 year revision rates for LPS are 86%; whereas VPS is 44%. When compared to VPS, overall LPS had a 2.5× risk of shunt revision and 3× risk of shunt obstruction [48]. Obstruction is the most common cause of shunt failure. The rate of infection and over drainage was comparable for both methods [48]. Additionally, patients with VPS had a shorter average length of stay and hospital costs as compared to those with LPS [69]. Due to the high rate of revision in LPS, El-Saadany et al., suggested that targeting patients more likely to benefit from LPS could enhance the effectiveness of the procedure. Predictors for increased success in LPS are patients with severe or fulminant CSF pressures or poor manometric response to repeated lumbar taps [70].
Recently a study has shown that ventriculo-pleural shunts can also be an effective means of CSF diversion in IIH as it is safe, easy and fast. The average time of procedure is shorter than the LPS placement. Patients undergoing this procedure should have a pulmonary function test to ensure pleura is healthy and has adequate absorption capacity. This prevents respiratory insufficiency and pleural effusions post-operation. Visual acuity showed significant improvement at 3, 6 and 12 month follow up.
Complications
Initially, CSF shunts were heralded as potentially superior to medical management due to their efficacy and low morbidity [71]. However, over time the reported complications and high revision rates have clouded the initial excitement. While it has a significant effect in resolving increased ICP and the related headaches and vision loss, headaches remain in a significant portion of patients. Sinclair et al. in their 10 year retrospective study found that 79% of patients still complained of headaches 2 years post-surgery. These headaches vary in nature and include low pressure headaches from over drainage in LPS procedures, migraine, and analgesic overuse.
The complication rate of LPS is widely variant in literature with reports ranging between 18 and 85% [71]. The most common complication of LPS is obstruction leading to failure. The rate of failure is cited as 86% by Mcgirt et al’s 42 patient 30 year retrospective study [48]. Obstructions commonly occur due to migration of the distal catheter. Another common complication of CSF shunts is over drainage of the CSF leading to nausea, vomiting, nuchal rigidity, visual disturbances and acquired Chiari I malformations. The use of programmable valves in VPS required less revisions over time and showed a statistically significant decreases in overall complications, overdrainage rates, and underdrainage rates [72, 73]. Infections are the complication of shunts that carry the highest risk of mortality. Risk of infection is increased by delayed timing of procedure scheduling or a prolonged duration of surgery. Infected shunts should be temporarily removed until infection resolves [70]. In-hospital mortality rates for shunt placement in IIH range between 0.1 and 0.5% of patients with majority having LPS versus VPS [69].
Venous Sinus Stenting
This topic is covered in more detail in a separate chapter. Over the last 20 years, it has become clear that most patients with IIH have stenosis along the transverse-sigmoid sinus junction (TSJ) , either bilaterally or in one dominant sinus [74]. After King et al. demonstrated a trans-venous pressure gradient in patients with IIH, Higgins et al. demonstrated symptomatic improvement in eight patients who underwent stenting of the stenosis [75–77].
First, a conventional cerebral angiogram measures the pressure gradient and a stent is only appropriate if there is difference of 8 mm Hg between the proximal transverse and distal sigmoid sinuses [78].
Venous sinus stenting is a relatively new procedure and researchers are still gathering data regarding how long the procedure manages IIH and what complications may occur over time. Stenting seems to address the underlying cause of the high pressure that characterizes IIH, but the exact mechanism remains controversial. It is important to remember that none of these surgical interventions (ONSF, shunt, stent) have been studied in a randomized trial.
The relationship of IIH to VSS and venous hypertension remains controversial. Although the majority of IIH patients harbor VSS, the degree of stenosis does not correlate with the clinical course, degree of VF loss or OP on LP [77]. Also, although venous sinus stenting can be performed in the setting of headache, it is not currently an effective treatment in the setting of vision loss like ONSF. However, it could be an option in patients who continue to have vision deterioration after ONSF.
The potential complications of cerebral angiography and stenting include both the possibility of bleeding into the brain or clotting around the stent. It is possible that the stent could move or that another narrowed area near the stent develops after it is in place [78].
Global Perspective
Based on current scientific literature, there is no clear optimum method for surgical intervention in IIH. Most studies have shown no significant difference when it comes to visual improvement, headache reduction, papilledema resolution and surgical morbidities. The choices currently lie between ONSF and CSF diversion with some variation on a shunt. Sinus stenting is achieving good results with a relatively low complication rate but there is insufficient evidence in the literature to confirm its absolute role. As such, a large, multicenter, randomized, physician-blinded, head-to-head trial comparing venous stenting, ONSF, and shunting is needed to compare the results and complications. As of now, many experts have their own preferences based on experience and institutional availability.