Animal Models for Chikungunya Virus and Zika Virus
Thomas E. Morrison University of Colorado School of Medicine, Aurora, CO, United States
Abstract
Chikungunya virus and Zika virus are mosquito-transmitted viruses of the genera Alphavirus and Flavivirus, respectively. In the last decade, both of these viruses have dramatically re-emerged, spreading to Asia, Europe, the Pacific Region, and the Americas. In each of these regions, these viruses have caused large outbreaks of acute and chronic disease in humans, including a devastating congenital syndrome in infants exposed to Zika virus infection in the womb. As part of the response to these ongoing public health crises, the development of robust animal models of human disease is major research priority. This chapter reviews the development and characterization of animal models being used to investigate the pathogenesis and immunity of these virus-induced diseases and for evaluating vaccines and therapeutics against chikungunya virus and Zika virus infection.
Keywords
Chikungunya virus; Zika virus; Animal model; Arbovirus; Pathology
Chikungunya Virus
Chikungunya virus (CHIKV) is a mosquito-transmitted alphavirus that causes explosive epidemics of a severe febrile illness characterized by debilitating polyarthralgia, polyarthritis, tenosynovitis, myalgia, headache, and rash (Weaver and Lecuit, 2015). The development of fever is temporally associated with viremia, which can reach up to 108–109 PFU or copies of viral RNA (vRNA) per ml of blood (Panning et al., 2008; Chow et al., 2011; Werneke et al., 2011; Kam et al., 2012). The joint pain and inflammation are usually symmetric and localized to joints in the arms and the legs such as the interphalangeal joints, wrists, knees, and ankles. The pain and inflammation in musculoskeletal tissues can last from a few days to several years; thus, there are clear acute, postacute, and chronic clinical stages (Simon et al., 2015). Atypical outcomes of CHIKV infection occur in neonates, the elderly, and in those with underlying medical comorbidities. These outcomes include encephalopathy and encephalitis, myocarditis, hepatitis, multiorgan failure, and death.
Since 2004, CHIKV has caused millions of disease cases in the Indian Ocean region and has emerged in new areas, including Europe, the Middle East, the Pacific region, and most recently, islands of the Caribbean (Leparc-Goffart et al., 2014; Morrison, 2014; Weaver and Forrester, 2015). The CHIKV outbreak in the Caribbean rapidly spread to surrounding areas and the virus has caused greater than 1.8 million cases in more than 45 countries and territories in the Western Hemisphere (Morrison, 2014). Although not typically life threatening, acute and chronic CHIKV disease are debilitating and the large epidemics have severe economic impacts. The re-emergence of CHIKV and global spread of the virus triggered renewed interest in the molecular mechanisms by which CHIKV, and related arthritogenic alphaviruses, cause acute and chronic disease. Thus, great progress has been made in the development and characterization of animal models of CHIKV disease (Table 1). These models are used to investigate mechanisms of CHIKV infection, pathogenesis, and immunity. In addition, the development of these models has been exploited for the evaluation of novel therapeutics and vaccines against CHIKV infection.
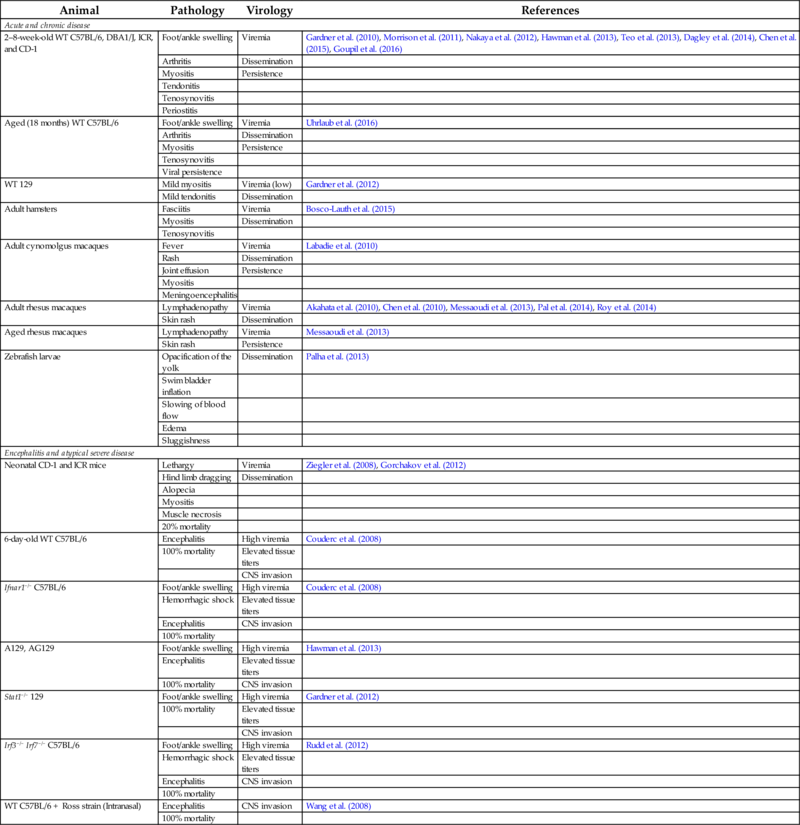
Table 1
| Animal | Pathology | Virology | References |
|---|---|---|---|
| Acute and chronic disease | |||
| 2–8-week-old WT C57BL/6, DBA1/J, ICR, and CD-1 | Foot/ankle swelling | Viremia | Gardner et al. (2010), Morrison et al. (2011), Nakaya et al. (2012), Hawman et al. (2013), Teo et al. (2013), Dagley et al. (2014), Chen et al. (2015), Goupil et al. (2016) |
| Arthritis | Dissemination | ||
| Myositis | Persistence | ||
| Tendonitis | |||
| Tenosynovitis | |||
| Periostitis | |||
| Aged (18 months) WT C57BL/6 | Foot/ankle swelling | Viremia | Uhrlaub et al. (2016) |
| Arthritis | Dissemination | ||
| Myositis | Persistence | ||
| Tenosynovitis | |||
| Viral persistence | |||
| WT 129 | Mild myositis | Viremia (low) | Gardner et al. (2012) |
| Mild tendonitis | Dissemination | ||
| Adult hamsters | Fasciitis | Viremia | Bosco-Lauth et al. (2015) |
| Myositis | Dissemination | ||
| Tenosynovitis | |||
| Adult cynomolgus macaques | Fever | Viremia | Labadie et al. (2010) |
| Rash | Dissemination | ||
| Joint effusion | Persistence | ||
| Myositis | |||
| Meningoencephalitis | |||
| Adult rhesus macaques | Lymphadenopathy | Viremia | Akahata et al. (2010), Chen et al. (2010), Messaoudi et al. (2013), Pal et al. (2014), Roy et al. (2014) |
| Skin rash | Dissemination | ||
| Aged rhesus macaques | Lymphadenopathy | Viremia | Messaoudi et al. (2013) |
| Skin rash | Persistence | ||
| Zebrafish larvae | Opacification of the yolk | Dissemination | Palha et al. (2013) |
| Swim bladder inflation | |||
| Slowing of blood flow | |||
| Edema | |||
| Sluggishness | |||
| Encephalitis and atypical severe disease | |||
| Neonatal CD-1 and ICR mice | Lethargy | Viremia | Ziegler et al. (2008), Gorchakov et al. (2012) |
| Hind limb dragging | Dissemination | ||
| Alopecia | |||
| Myositis | |||
| Muscle necrosis | |||
| 20% mortality | |||
| 6-day-old WT C57BL/6 | Encephalitis | High viremia | Couderc et al. (2008) |
| 100% mortality | Elevated tissue titers | ||
| CNS invasion | |||
| Ifnar1−/− C57BL/6 | Foot/ankle swelling | High viremia | Couderc et al. (2008) |
| Hemorrhagic shock | Elevated tissue titers | ||
| Encephalitis | CNS invasion | ||
| 100% mortality | |||
| A129, AG129 | Foot/ankle swelling | High viremia | Hawman et al. (2013) |
| Encephalitis | Elevated tissue titers | ||
| 100% mortality | CNS invasion | ||
| Stat1−/− 129 | Foot/ankle swelling | High viremia | Gardner et al. (2012) |
| 100% mortality | Elevated tissue titers | ||
| CNS invasion | |||
| Irf3−/− Irf7−/− C57BL/6 | Foot/ankle swelling | High viremia | Rudd et al. (2012) |
| Hemorrhagic shock | Elevated tissue titers | ||
| Encephalitis | CNS invasion | ||
| 100% mortality | |||
| WT C57BL/6 + Ross strain (Intranasal) | Encephalitis | CNS invasion | Wang et al. (2008) |
| 100% mortality | |||
Animal Models of CHIKV Musculoskeletal Disease
Acute CHIKV Disease
As discussed here and in Chapter 3, CHIKV infection in humans commonly causes a rheumatological disease characterized by arthralgia, arthritis, tenosynovitis, myositis, and myalgia. Accordingly, efforts have been made to develop and characterize animal models of acute CHIKV musculoskeletal disease, with CHIKV infection of WT C57BL/6 mice the most extensively characterized model to date. Inoculation of 2–8-week-old WT C57BL/6 mice subcutaneously in the footpad with a variety of CHIKV strains produces disease signs with similarities to the human disease including metatarsal swelling of the inoculated foot, a mixed inflammatory cell arthritis in the foot and ankle, tenosynovitis, myositis, and periostitis (Gardner et al., 2010; Morrison et al., 2011; Teo et al., 2013; Goupil et al., 2016). Consistent with our knowledge of joint disease in humans infected with CHIKV or a related arthritogenic alphavirus, further characterization of the inflammatory cell infiltrate in joint-associated tissues identified natural killer cells, neutrophils, monocytes, macrophages, and T lymphocytes as the predominant cellular infiltrates (Gardner et al., 2010; Morrison et al., 2011). Furthermore, analysis of CHIKV-infected feet by microcomputed tomography revealed that CHIKV infection induces the loss of trabecular and cortical bone, which has been observed in some CHIKV-infected patients (Malvy et al., 2009), by as early as 3 days postinfection (dpi) (Chen et al., 2015). It should be noted that these studies were conducted in 25-day-old WT C57BL/6 mice (Mus musculus); loss of trabecular or cortical bone mass was not detected in 8-week-old WT C57BL/6 mice inoculated with a different strain of CHIKV (Goupil et al., 2016). Nevertheless, both studies consistently observed active osteoclasts in bone and joint tissues of CHIKV-infected mice, suggesting that osteoclastic bone resorption is a component of CHIKV disease. In addition to these findings, gene expression profiling revealed shared gene expression signatures in RNA samples collected from the joints of mice with CHIKV- or collagen-induced arthritis, a mouse model of rheumatoid arthritis (RA), as well as synovial tissues from humans with RA (Nakaya et al., 2012). Virologically, CHIKV-infected WT C57BL/6 mice develop a high titer viremia lasting for 4–5 days or 10 days when measured by plaque assay for infectious virus or RT-PCR for vRNA, respectively (Gardner et al., 2010; Teo et al., 2013). In addition, the virus replicates in several peripheral tissues, with the highest viral titers per gram found in joint-associated tissue (Gardner et al., 2010; Morrison et al., 2011; Hawman et al., 2013). Although not as extensively characterized, similar observations have been made in CHIKV-infected outbred Institute for Cancer Research (ICR) and CD-1 mice (Ziegler et al., 2008; Gardner et al., 2014), WT 129 mice (Gardner et al., 2012), and DBA/1J mice (Dagley et al., 2014). However, even when inoculated subcutaneously in the footpad, WT 129 mice did not develop swelling of the inoculated foot and inflammatory cell infiltration of foot tissues appeared to be milder than reported for CHIKV-infected WT C57BL/6 mice (Gardner et al., 2012), suggesting that the underlying genetics of the mouse strain used may influence the development of acute CHIKV musculoskeletal disease.
Limited studies have evaluated acute CHIKV infection in other vertebrate species, including birds, hamsters (Mesocricetus auratus), and zebrafish (Danio rerio) (Palha et al., 2013; Bosco-Lauth et al., 2015, 2016). Six-month-old hamsters inoculated with CHIKV subcutaneously in the ventral abdomen developed viremia and had detectable viral loads in a variety of organs, including heart, spleen, skin, and muscle, up to 4 dpi. Histologically, these animals displayed hind limb fasciitis, necrotizing myositis, and tenosynovitis during the acute phase (Bosco-Lauth et al., 2015), similar to observations in CHIKV-infected mice. CHIKV infection of zebrafish larvae (3 days postfertilization) via intravenous injection results in only mild and transient disease signs such as opacification of the yolk, swim bladder inflation, slowing of blood flow, edema, and sluggishness in a subset of animals (Palha et al., 2013). However, CHIKV appears to replicate and disseminate robustly in these animals with high viral titers detected in most organs at 24–48 h postinoculation. Due to the transparency of zebrafish larvae, infection with recombinant viruses that express fluorescent proteins, such as GFP, allows live-imaging of CHIKV infection and other in vivo imaging techniques to characterize individual CHIKV-infected cells (Palha et al., 2013). In addition, the existence of a variety of tools to manipulate gene expression in zebrafish facilitates mechanistic experiments.
In addition to mouse genetics, the outcome of CHIKV infection in mice is age dependent. WT C57BL/6 mice inoculated intradermally with CHIKV display age-dependent mortality, with 6-day-old mice succumbing to the infection, ~ 50% of 9-day-old mice succumbing to the infection, and no morality observed in mice that were 12 days old or older at the time of CHIKV inoculation (Couderc et al., 2008; Morrison et al., 2011; Werneke et al., 2011). In addition to mortality, the severity of virus-induced foot swelling and tissue injury is influenced by age. For example, in comparison with 12-week-old WT C57BL/6 mice, which develop milder musculoskeletal disease compared with younger mice (e.g., 2–4-week-old mice), CHIKV-induced foot swelling in 18-month-old WT C57BL/6 mice was more severe (Uhrlaub et al., 2016). Consistent with studies showing that CHIKV-infected elderly humans harbor higher viral loads (Werneke et al., 2011), older mice have a prolonged viremia and elevated viral loads in tissues compared with adult mice (Uhrlaub et al., 2016). Collectively, these data suggest that the increased susceptibility of neonates and older adults to more severe CHIKV disease can be modeled and therefore investigated in mice.
In summary, the WT C57BL/6 mouse model, as well as infection studies in other mouse strains, is now extensively employed by numerous research laboratories to investigate pathogenic mechanisms of acute CHIKV musculoskeletal disease, to study innate and adaptive immunity to CHIKV infection, to define viral virulence determinants, to evaluate the influence of mosquito saliva and mosquito-based transmission on infection, and to evaluate candidate vaccines or therapeutics for efficacy against CHIKV disease.
In addition to studies in mice, acute CHIKV infection and disease has been investigated in cynomolgus (Macaca fascicularis) and rhesus (Macaca mulatta) macaques (Broeckel et al., 2015). Adult (3–5 years old) cynomolgus macaques inoculated intradermally or intravenously with CHIKV develop some disease signs consistent with human disease, such as a high fever that lasts several days, a skin rash, swelling in wrist and ankle joints, and meningoencephalitis, which can occur rarely in infected humans (Labadie et al., 2010; Gerardin et al., 2016). Of note, only animals inoculated with high doses of virus (> 107 PFU) developed clinical signs of joint swelling and meningoencephalitis (Labadie et al., 2010). CHIKV-infected cynomolgus macaques develop a high titer viremia associated with monocytopenia, lymphopenia, thrombocytopenia, and granulocytosis. In addition, infectious virus and viral RNA were detected in lymphoid, joint, and muscle tissue as well as the liver at early times postinfection (2–8 days) (Labadie et al., 2010). Severe histiocytosis was evident in the spleen and lymph nodes, whereas focal inflammation occurred in skeletal muscle tissue. In some animals with joint swelling, mononuclear cell infiltration of synovial tissues was observed (Labadie et al., 2010). Using radiotelemetry to monitor physiologic responses, cynomolgus macaques inoculated subcutaneously with 105 PFU of CHIKV were shown to experience increased core body temperature for 1–2 days followed by periods of hypothermia out to 2 weeks postinfection (Roy et al., 2014). Telemetric monitoring also revealed an elevated heart rate lasting 7–10 days post-CHIKV infection (Roy et al., 2014). Finally, as will be discussed in more detail later, long-term persistent CHIKV infection is detectable in multiple tissues of cynomolgus macaques (Labadie et al., 2010), suggesting that this model can be exploited to investigate chronic CHIKV disease pathogenesis.
Several studies have evaluated CHIKV infection in rhesus macaques, including aged and pregnant animals. Adult rhesus macaques (3–13 years old) inoculated intravenously with high doses of CHIKV (107–1010 PFU) develop viremia lasting 3–4 days, fever, transient lymphopenia, lymphadenopathy, and in some animals, a skin rash (Akahata et al., 2010; Messaoudi et al., 2013). Notably, pregnant rhesus macaques infected with CHIKV displayed fever for a much longer duration (up to 17–19 days) as well as increased joint temperature and joint swelling (Chen et al., 2010). Histopathological and virological analysis of tissues from nonpregnant adults at 5–6 weeks postinfection showed an absence of joint inflammation and virus in various tissues (Messaoudi et al., 2013), suggesting an absence of chronic disease and infection in adult rhesus macaques. In contrast, viral RNA persisted in the spleen of CHIKV-infected aged (> 17 years old) rhesus macaques (Messaoudi et al., 2013). CHIKV persistence in the spleen of aged rhesus macaques was virus strain-dependent and was associated with defects in CHIKV-specific immunity (Messaoudi et al., 2013). These findings indicate that the rhesus macaque model can be utilized to investigate mechanisms of protective immunity against CHIKV infection. The dissemination and tissue distribution of CHIKV infection during the acute phase has been more extensively investigated following subcutaneous inoculation of the virus in both arms of adult rhesus macaques (6–13 years old) (Pal et al., 2014). At 7 dpi, CHIKV RNA was detected in a variety of joints (wrist, elbow, knee, and ankle), muscles, and secondary lymphoid tissue. In addition, low levels of CHIKV RNA were detected in organs including the heart, lungs, and kidney. Similar findings were made in CHIKV-infected pregnant rhesus macaques, with viral RNA detected at 21 dpi in maternal secondary lymphoid tissues, joint-associated connective tissue, and skeletal muscle tissue of some animals (Chen et al., 2010). Collectively, these data indicate that CHIKV readily spreads from the site of inoculation in rhesus macaques; thus, this model can be used to investigate viral determinants of replication and dissemination and to test therapeutic interventions that antagonize viral dissemination and reduce viral burdens in musculoskeletal and other tissues.
Chronic CHIKV Disease
In many patients diagnosed with CHIKV infection, pain and inflammation in joint-associated tissues persist for months to years (Borgherini et al., 2008; Gerardin et al., 2011; Schilte et al., 2013). The mechanisms of chronic CHIKV disease pathogenesis are not well understood. However, experiments performed in cynomolgus macaques were some of the first to provide evidence of persistent CHIKV infection (Labadie et al., 2010). Infectious virus, viral RNA (detected by qRT-PCR and ISH), and viral antigen were detected in joint-associated tissue, muscle tissue, and secondary lymphoid tissue 1.5–3 months after inoculation (Labadie et al., 2010). In lymphoid tissues, the viral antigen localized to CD68+ macrophages, suggesting that these cells serve as a reservoir for persistent CHIKV infection. Although persistent CHIKV infection was not associated with signs of chronic joint disease in this model, the findings support the hypothesis that chronic CHIKV disease is caused by inflammatory processes driven by viral persistence and support the use of this model to investigate mechanisms of chronic CHIKV infection. In contrast to cynomolgus macaques, persistent CHIKV infection was not detected in secondary lymphoid tissues or joint-associated tissues of adult rhesus macaques. However, as mentioned earlier, viral RNA was detected at late times postinfection in the spleen of aged rhesus macaques (Messaoudi et al., 2013).
Mouse models also have been employed to investigate mechanisms of chronic CHIKV infection and disease. Following infection of WT C57BL/6 mice, inoculated subcutaneously in the footpad with various isolates of CHIKV, viral RNA and infectious virus can be detected in joint-associated tissues for months postinfection (Hawman et al., 2013; Poo et al., 2014; Uhrlaub et al., 2016). The persistence of CHIKV in this tissue is associated with persistent synovitis and myositis (Hawman et al., 2013; Poo et al., 2014; Uhrlaub et al., 2016) and an inflammatory gene expression signature (Poo et al., 2014), suggesting that chronic CHIKV infection promotes joint inflammation. Thus, the C57BL6 mouse model can be used to study viral and host factors, such as viral determinants and host immune responses (Hawman et al., 2013, 2016; Poo et al., 2014; Seymour et al., 2015; Uhrlaub et al., 2016), that influence the establishment of persistent infection in joints and the development of chronic arthritis.
Animal Models of CHIKV Encephalitis and Other Severe Outcomes
CHIKV infection is associated with more severe diseases, including encephalitis, in neonates (Gerardin et al., 2016). The rate of infection of neonates born to viremic mothers and exposed to the virus during birth can reach 50% (Gerardin et al., 2008). As discussed, WT C57BL/6 neonatal mice develop lethal encephalitis following infection with CHIKV, with viral antigen detected in leptomeningial cells (Couderc et al., 2008). Neonatal ICR and CD-1 mice (2–3 days old) inoculated subcutaneously with CHIKV also develop severe morbidity; however, the mortality rate in these mice is lower than that observed in 6-day-old WT C57BL/6 mice (10%–20% vs 100%, respectively) (Couderc et al., 2008; Ziegler et al., 2008). CHIKV infection also disseminates to the central nervous system (CNS) of adult Ifnar1−/− C57BL/6 mice, and the elevated viral loads in this tissue are associated with severe disease signs and death of the animal (Couderc et al., 2008). Similar observations have been made in adult 129 mice lacking Ifnar1 (A129), Ifnar1 and Ifngr1 (AG129), or Stat1 (Gardner et al., 2012; Gorchakov et al., 2012). Further studies in Ifnar1−/− C57BL/6 mice, as well as Irf3−/−;Irf7−/− C57BL/6 mice, showed that CHIKV infection in these animals is associated with features of hemorrhagic shock, including vasculitis, hemorrhage, and thrombocytopenia (Rudd et al., 2012). In addition to these models, studies performed to evaluate the outcome of CHIKV infection with different viral strains by different routes found that intranasal inoculation of 5-week-old WT C57BL/6 mice with the Ross strain, which has been extensively passaged in neonatal mice, resulted in high viral titers in the brain, inflammation and necrosis in CNS tissues, signs of CNS disease, and death of the animal (Wang et al., 2008). These findings were mouse age- and mouse strain-dependent, as 10-week-old WT C57BL/6 mice and 5- or 10-week-old NIH Swiss Webster mice inoculated intranasally with the Ross strain showed minimal morbidity and no mortality. Collectively, neonatal mice, adult mice with genetic lesions in essential components of the type I IFN pathway, and intranasal inoculation of the Ross strain into 5-week-old WT C57BL/6 mice provide model systems to investigate mechanisms of severe, atypical outcomes associated with CHIKV infection as well as lethal challenge models for evaluating the protective capacity of experimental vaccines and antiviral therapeutics.
Zika Virus
Zika virus (ZIKV) was first isolated in 1947 from the blood of a sentinel rhesus monkey in the Zika forest of Uganda (Dick et al., 1952). Since the discovery of ZIKV, reported cases of infection in humans were rare as ZIKV infection typically was associated with relatively mild symptoms (Musso and Gubler, 2016). However, the magnitude and severity of the 2007 ZIKV outbreak in Micronesia (Duffy et al., 2009), the 2013–14 outbreak in French Polynesia (Cao-Lormeau et al., 2014, 2016), followed by the 2015-present outbreak in the Americas (Hennessey et al., 2016; Mlakar et al., 2016; Schuler-Faccini et al., 2016), in which ZIKV infection was associated with more severe outcomes including Guillain-Barré syndrome in adults and microcephaly in infants, prompted the World Health Organization (WHO) to declare a Public Health Emergency of International Concern in early 2016. These dramatic events led researchers to rapidly engage in efforts to develop animal models of ZIKV replication and pathogenesis (Table 2). Despite the short time, an intense effort by many research teams has resulted in the development and characterization of several different animal models that can be used to investigate mechanisms of ZIKV replication and spread, ZIKV pathogenesis in adults and developing fetuses, immunological responses to ZIKV, and to evaluate novel therapeutics and vaccines for efficacy against ZIKV infection.
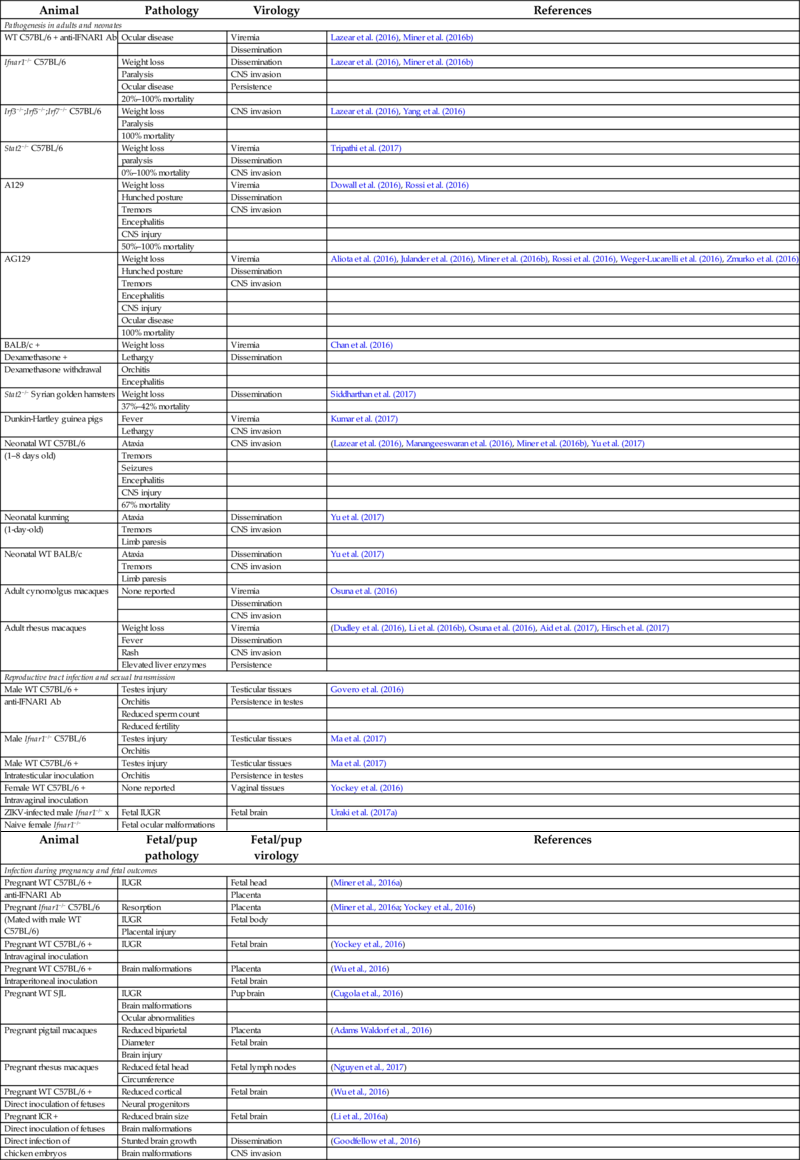
Table 2
| Animal | Pathology | Virology | References |
|---|---|---|---|
| Pathogenesis in adults and neonates | |||
| WT C57BL/6 + anti-IFNAR1 Ab | Ocular disease | Viremia | Lazear et al. (2016), Miner et al. (2016b) |
| Dissemination | |||
| Ifnar1−/− C57BL/6 | Weight loss | Dissemination | Lazear et al. (2016), Miner et al. (2016b) |
| Paralysis | CNS invasion | ||
| Ocular disease | Persistence | ||
| 20%–100% mortality | |||
| Irf3−/−;Irf5−/−;Irf7−/− C57BL/6 | Weight loss | CNS invasion | Lazear et al. (2016), Yang et al. (2016) |
| Paralysis | |||
| 100% mortality | |||
| Stat2−/− C57BL/6 | Weight loss | Viremia | Tripathi et al. (2017) |
| paralysis | Dissemination | ||
| 0%–100% mortality | CNS invasion | ||
| A129 | Weight loss | Viremia | Dowall et al. (2016), Rossi et al. (2016) |
| Hunched posture | Dissemination | ||
| Tremors | CNS invasion | ||
| Encephalitis | |||
| CNS injury | |||
| 50%–100% mortality | |||
| AG129 | Weight loss | Viremia | Aliota et al. (2016), Julander et al. (2016), Miner et al. (2016b), Rossi et al. (2016), Weger-Lucarelli et al. (2016), Zmurko et al. (2016) |
| Hunched posture | Dissemination | ||
| Tremors | CNS invasion | ||
| Encephalitis | |||
| CNS injury | |||
| Ocular disease | |||
| 100% mortality | |||
| BALB/c + | Weight loss | Viremia | Chan et al. (2016) |
| Dexamethasone + | Lethargy | Dissemination | |
| Dexamethasone withdrawal | Orchitis | ||
| Encephalitis | |||
| Stat2−/− Syrian golden hamsters | Weight loss | Dissemination | Siddharthan et al. (2017) |
| 37%–42% mortality | |||
| Dunkin-Hartley guinea pigs | Fever | Viremia | Kumar et al. (2017) |
| Lethargy | CNS invasion | ||
| Neonatal WT C57BL/6 | Ataxia | CNS invasion | (Lazear et al. (2016), Manangeeswaran et al. (2016), Miner et al. (2016b), Yu et al. (2017) |
| (1–8 days old) | Tremors | ||
| Seizures | |||
| Encephalitis | |||
| CNS injury | |||
| 67% mortality | |||
| Neonatal kunming | Ataxia | Dissemination | Yu et al. (2017) |
| (1-day-old) | Tremors | CNS invasion | |
| Limb paresis | |||
| Neonatal WT BALB/c | Ataxia | Dissemination | Yu et al. (2017) |
| Tremors | CNS invasion | ||
| Limb paresis | |||
| Adult cynomolgus macaques | None reported | Viremia | Osuna et al. (2016) |
| Dissemination | |||
| CNS invasion | |||
| Adult rhesus macaques | Weight loss | Viremia | (Dudley et al. (2016), Li et al. (2016b), Osuna et al. (2016), Aid et al. (2017), Hirsch et al. (2017) |
| Fever | Dissemination | ||
| Rash | CNS invasion | ||
| Elevated liver enzymes | Persistence | ||
| Reproductive tract infection and sexual transmission | |||
| Male WT C57BL/6 + | Testes injury | Testicular tissues | Govero et al. (2016) |
| anti-IFNAR1 Ab | Orchitis | Persistence in testes | |
| Reduced sperm count | |||
| Reduced fertility | |||
| Male Ifnar1−/− C57BL/6 | Testes injury | Testicular tissues | Ma et al. (2017) |
| Orchitis | |||
| Male WT C57BL/6 + | Testes injury | Testicular tissues | Ma et al. (2017) |
| Intratesticular inoculation | Orchitis | Persistence in testes | |
| Female WT C57BL/6 + | None reported | Vaginal tissues | Yockey et al. (2016) |
| Intravaginal inoculation | |||
| ZIKV-infected male Ifnar1−/− x | Fetal IUGR | Fetal brain | Uraki et al. (2017a) |
| Naive female Ifnar1−/− | Fetal ocular malformations | ||
| Animal | Fetal/pup pathology | Fetal/pup virology | References |
| Infection during pregnancy and fetal outcomes | |||
| Pregnant WT C57BL/6 + | IUGR | Fetal head | (Miner et al., 2016a) |
| anti-IFNAR1 Ab | Placenta | ||
| Pregnant Ifnar1−/− C57BL/6 | Resorption | Placenta | (Miner et al., 2016a; Yockey et al., 2016) |
| (Mated with male WT C57BL/6) | IUGR | Fetal body | |
| Placental injury | |||
| Pregnant WT C57BL/6 + | IUGR | Fetal brain | (Yockey et al., 2016) |
| Intravaginal inoculation | |||
| Pregnant WT C57BL/6 + | Brain malformations | Placenta | (Wu et al., 2016) |
| Intraperitoneal inoculation | Fetal brain | ||
| Pregnant WT SJL | IUGR | Pup brain | (Cugola et al., 2016) |
| Brain malformations | |||
| Ocular abnormalities | |||
| Pregnant pigtail macaques | Reduced biparietal | Placenta | (Adams Waldorf et al., 2016) |
| Diameter | Fetal brain | ||
| Brain injury | |||
| Pregnant rhesus macaques | Reduced fetal head | Fetal lymph nodes | (Nguyen et al., 2017) |
| Circumference | |||
| Pregnant WT C57BL/6 + | Reduced cortical | Fetal brain | (Wu et al., 2016) |
| Direct inoculation of fetuses | Neural progenitors | ||
| Pregnant ICR + | Reduced brain size | Fetal brain | (Li et al., 2016a) |
| Direct inoculation of fetuses | Brain malformations | ||
| Direct infection of | Stunted brain growth | Dissemination | (Goodfellow et al., 2016) |
| chicken embryos | Brain malformations | CNS invasion | |
Animal Models of ZIKV Infection of Adults and Neonates
ZIKV infection of adult humans often results in an asymptomatic infection or more rarely the development of a mild febrile illness with accompanying joint pain, rash, and conjunctivitis. ZIKV infection in some adults has been associated with severe neurological disease (Carteaux et al., 2016; Dirlikov et al., 2016; Soares et al., 2016). Building on extensive data indicating that mice with genetic deficiencies in the type I IFN pathway display enhanced susceptibility to infection with a variety of arboviruses, initial studies focused on evaluating the capacity of immunocompromised mice to support ZIKV replication. Infection of immunocompetent CD-1, WT 129Sv/Ev, and WT C57BL/6 mice with ZIKV resulted in no detectable disease signs and little to no detectable infectious virus or viral RNA in tissues (Dowall et al., 2016; Lazear et al., 2016; Rossi et al., 2016). In contrast, mice lacking the Ifnar1 gene, including A129 mice and Ifnar1−/− C57BL/6 mice, or mice triply deficient in Irf3, Irf5, and Irf7 (Irf3−/− Irf5−/− Irf7−/− TKO) develop disease signs including hunched posture, ruffled fur, and hind limb weakness and paralysis, and succumb following intraperitoneal, subcutaneous in the footpad, subcutaneous in the leg, or intravenous inoculation of ZIKV (Dowall et al., 2016; Lazear et al., 2016; Rossi et al., 2016; Yang et al., 2016). In addition, consistent with the findings that ZIKV evades cellular antiviral responses by NS5-mediated degradation of human, but not mouse, STAT2 (a transcription factor that regulates induction of IFN-stimulated genes) (Grant et al., 2016), ZIKV infection of Stat2−/− C57BL/6 mice leads to neurological disease signs, weight loss, and mortality (Tripathi et al., 2017). Notably, the severity of disease signs and mortality in Stat2−/− mice was virus strain-dependent, with African ZIKV strains causing more severe morbidity and mortality compared with Asian ZIKV strains.
ZIKV-infected A129 mice of all tested ages develop signs of disease (Dowall et al., 2016; Rossi et al., 2016). However, more severe signs of disease and death were found to be age-dependent, with 100% of 3-week-old, 50% of 5-week-old, and 0% of 11-week-old A129 mice succumbing to infection (Rossi et al., 2016). Consistent with these data, the outcome of ZIKV infection in Ifnar1−/− C57BL/6 mice also is age-dependent, with 3-, 4-, and 6-month-old mice displaying enhanced survival compared with 5-6-week-old mice (Lazear et al., 2016).
Mice lacking both the type I and type II IFN receptors (AG129) also show severe disease following ZIKV infection (Aliota et al., 2016; Julander et al., 2016; Miner et al., 2016b; Rossi et al., 2016; Weger-Lucarelli et al., 2016; Zmurko et al., 2016). By intraperitoneal, intradermal, and subcutaneous inoculation routes, ZIKV infection is uniformly fatal in AG129 mice (Aliota et al., 2016; Rossi et al., 2016; Weger-Lucarelli et al., 2016). In fact, subcutaneous inoculation of as little as 1 PFU of ZIKV results in 100% lethality in AG129 mice (Aliota et al., 2016). The severe outcomes of ZIKV infection in A129 and AG129 mice, including tremors, ataxia, paralysis, and conjunctivitis, are associated with extensive pathology in the brain as well as high viral loads in numerous tissues, particularly the brain, the spleen, and the testes (Aliota et al., 2016; Dowall et al., 2016; Rossi et al., 2016; Weger-Lucarelli et al., 2016; Zmurko et al., 2016).
ZIKV infection of adults often results in conjunctivitis (Cerbino-Neto et al., 2016; Jimenez Corona et al., 2016; Meltzer et al., 2016) and uveitis in an adult patient diagnosed with ZIKV infection has been described (Furtado et al., 2016). In addition, ZIKV RNA was detected in lacrimal fluid of infected rhesus macaques (Li et al., 2016b). Ifnar1−/− mice infected with ZIKV develop conjunctivitis and panuveitis, and these disease manifestations were associated with ZIKV infection in the eye (Miner et al., 2016b). Thus, Ifnar1−/− mice also may be useful for investigating eye disease associated with ZIKV infection.
Building on findings indicating that immunosuppressed humans are more susceptible to flavivirus infections, one group evaluated ZIKV infection in experimentally immunosuppressed adult BALB/c mice (Chan et al., 2016). For this model, mice were treated with the immunosuppressant dexamethasone for 3 days prior to intraperitoneal inoculation of ZIKV (strain PRVABC59), and the mice continued to receive daily dexamethasone out to 9 days postinfection. ZIKV infection of dexamethasone-treated mice resulted in mild weight loss, viremia, and a disseminated infection with viral RNA and viral antigen detected in a variety of tissues. The withdrawal of dexamethasone 9 days after infection led to rapid deterioration of the mice, which was more severe in males compared with females, characterized by rapid weight loss and the development of disease signs including lethargy and ruffled fur (Chan et al., 2016). The deterioration of these mice postdexamethasone withdrawal was associated with prominent inflammation and injury in the brain, kidney, and testes.
Researchers also have evaluated the use of hamsters and guinea pigs as model systems to study ZIKV replication and pathogenesis. Due to the inability of ZIKV NS5 to antagonize mouse STAT2 (Grant et al., 2016), as described earlier, ZIKV infection was assessed in Stat2-deficient Syrian golden hamsters (Siddharthan et al., 2017). Following subcutaneous inoculation with a Malaysian strain of ZIKV, ~ 40% of adult Stat2-deficient hamsters succumbed to infection by 21 dpi. ZIKV infection was detected in a variety of tissues including the CNS, kidneys, and testes. In guinea pigs, subcutaneous inoculation with a Puerto Rican strain of ZIKV led to the development of mild disease signs including fever and lethargy (Kumar et al., 2017). At early times postinoculation (days 1–5), a low level viremia was detected, and viral RNA was detectable in the brain. Thus, these animals may have utility as alternative small animal model systems for the study of ZIKV replication and pathogenesis in vivo.
In addition to adult animals, ZIKV infection also has been studied in neonatal mice (Lazear et al., 2016; Manangeeswaran et al., 2016; Miner et al., 2016b; Yu et al., 2017). In contrast to most adult immunocompetent mice, infection of 7–8-day-old WT C57BL/6 mice by intraperitoneal or subcutaneous injection of ZIKV resulted some lethality as well as pathology in the CNS (Lazear et al., 2016; Miner et al., 2016b), indicating that ZIKV-induced disease in WT C57BL/6 mice is mouse age-dependent. In additional studies, subcutaneous inoculation of 1-day-old WT C57BL/6 mice with a Puerto Rican strain of ZIKV (PRVABC59) was found to result in generally nonfatal neurological disease characterized by kinetic tremors, ataxia, and seizures that developed approximately 2 weeks postinfection (Manangeeswaran et al., 2016). Similar outcomes were observed following infection of 1-day-old WT C57BL/6 mice via multipoint subcutaneous inoculation (Yu et al., 2017). These disease signs were associated with ZIKV infection in the CNS, neurodegeneration in the cerebellum, and infiltration of brain tissue with CD8+ and CD4+ T cells (Manangeeswaran et al., 2016; Yu et al., 2017). In comparison with WT C57BL/6 mice, ZIKV infection of 1-day-old Kunming or BALB/c mice via multipoint subcutaneous inoculation resulted in less severe weight loss and neurological disease signs (Yu et al., 2017). Thus, ZIKV infection of neonatal mice could be exploited to define mediators of pathology and, due to the high survival rate, may permit the study of long-term sequela associated with ZIKV infection of the immature CNS.
Nonhuman primates (NHPs) also have been used to evaluate basic aspects of ZIKV infection and pathogenesis (Dudley et al., 2016; Li et al., 2016b; Osuna et al., 2016). Thus far, researchers have characterized ZIKV infection in pregnant and nonpregnant rhesus macaques as well as cynomolgus macaques. In each study, contemporary ZIKV strains were inoculated subcutaneously, at doses comparable to viral doses inoculated by infected mosquitoes, and a variety of clinical, virological, and immunological parameters were assessed. Inoculation of rhesus macaques with an Asian lineage ZIKV strain from French Polynesia (H/FP/2013) resulted in mild weight loss, development of a mild rash around the injection site, and elevated serum creatine kinase and alanine aminotransferase in some animals, but no other clinical disease signs (Dudley et al., 2016). Although weight loss and rash were not observed across all studies, elevated liver enzymes at early times postinfection appear to be a consistent feature of ZIKV infection of rhesus macaques (Dudley et al., 2016; Li et al., 2016b; Osuna et al., 2016). In some studies, infection also resulted in elevated body temperature for 1–10 days postinfection (Li et al., 2016b; Osuna et al., 2016). Consistently, ZIKV-infected rhesus macaques develop plasma viremia that peaks 2–6 days postinfection and becomes generally undetectable by day 10. ZIKV RNA was detected in urine, saliva, and the cerebrospinal fluid of some animals across all studies, suggesting that a variety of tissues are infected. ZIKV RNA also was detected in the seminal fluid and vaginal secretions, somewhat sporadically, of some animals (Dudley et al., 2016; Osuna et al., 2016). Notably, ZIKV-infected pregnant rhesus macaques display persistent viremia (based on RT-PCR analysis) (Dudley et al., 2016). As there also is evidence of persistent viremia in pregnant women (Driggers et al., 2016), these findings suggest that ZIKV infection of pregnant rhesus macaques closely mimics at least some features of ZIKV infection of pregnant women. Using various approaches, including RNAscope in situ hybridization for the ZIKV genome, immunohistochemistry with a pan-flavivirus monoclonal antibody, and RT-PCR analysis of tissue RNA, ZIKV infection was detected in a variety of tissues of rhesus and cynomolgus macaques including secondary lymphoid organs, tissues in the male reproductive tract, the intestines, and the CNS (Li et al., 2016b; Osuna et al., 2016). Due to the prolonged viremia detected in infected pregnant women and persistence of ZIKV in semen from infected men (see below), ZIKV persistence in tissue sanctuaries also has been assessed in experimentally infected rhesus macaques (Aid et al., 2017; Hirsch et al., 2017). ZIKV RNA and replication-competent virus was detected in CSF at days 21 and 42 after infection, well beyond the clearance of virus in the plasma (Aid et al., 2017). In addition, ZIKV RNA and replication-competent virus was detectable in lymph nodes as late as day 72 postinfection (Aid et al., 2017). In a separate study, ZIKV RNA also was detected at late time points (28–35 dpi) in lymphoid, neuronal, and musculoskeletal tissues of infected rhesus macaques (Hirsch et al., 2017).
These initial studies support the use of rhesus and cynomolgus macaques as model systems to enhance our understanding of the cellular and tissue tropism of ZIKV infection, and the potential consequences of viral persistence. In addition, infected rhesus macaques developed ZIKV-specific humoral and cell-mediated immune responses (Dudley et al., 2016; Li et al., 2016b; Osuna et al., 2016; Aid et al., 2017; Hirsch et al., 2017), and were protected from rechallenge with homologous and heterologous viruses (Dudley et al., 2016; Osuna et al., 2016), indicating that this model will be useful for the evaluation of ZIKV immunity.
Collectively, the development and characterization of these animal models provide valuable systems to investigate ZIKV replication, cellular and tissue tropism, viral virulence determinants, and pathogenesis as well as for evaluating new therapeutics and vaccines for efficacy against ZIKV infection.
Modeling of Sexual Transmission of ZIKV
In addition to transmission by mosquitoes, sexual transmission of ZIKV has been reported (Foy et al., 2011; Musso et al., 2015; D'Ortenzio et al., 2016; Davidson et al., 2016; Deckard et al., 2016; Hills et al., 2016). In many of these reported cases, transmission has occurred from infected male to female partners. The increased frequency of sexual transmission from infected males to their sexual partners may be due to long-term persistence of ZIKV in testes and semen (Atkinson et al., 2016; Hills et al., 2016; Mansuy et al., 2016). In addition, hematospermia and prostatitis have been reported in ZIKV-infected men (Foy et al., 2011; Musso et al., 2015; Torres et al., 2016). Mouse models have been used for detailed investigation of ZIKV infection in the male reproductive tract (Govero et al., 2016; Ma et al., 2017; Uraki et al., 2017b). In some of these studies, male WT C57BL/6 mice were treated with a single dose of a blocking anti-IFNAR1 antibody followed by inoculation of a mouse-adapted strain of ZIKV subcutaneously in the footpad. The ZIKV strain Dakar 41519 was adapted to mice by passaging the virus in Rag1−/− mice (Govero et al., 2016). In this system, ZIKV infection was detected in numerous cell types of the male reproductive tract including spermatogonia, spermatocytes, mature sperm, and Sertoli cells, and ZIKV infection in the male reproductive tract persisted for at least 3 weeks. Infection at these sites was associated with inflammation and injury in testicular tissues and decreased male fertility (Govero et al., 2016). Similar studies were performed in male Ifnar1−/− C57BL/6 mice inoculated either subcutaneously of intraperitoneally with contemporary ZIKV strains (Ma et al., 2017; Uraki et al., 2017b). In this model, ZIKV infection also led to inflammation and injury in tissues of the male reproductive tract, including the testes and epididymis, and ZIKV infection was detected in spermatogonia and testicular peritubular-myoid cells. In addition, Ifnar1−/− mice that survived acute ZIKV infection displayed severe damage in the testes out to day 60 postinfection (Ma et al., 2017). Similar outcomes, including testicular inflammation and injury out to 30 days postinfection, were observed following direct intratesticular inoculation of ZIKV, but not DENV, into WT C57BL/6 mice (Ma et al., 2017). The development of these models allows for further investigation of ZIKV pathogenesis and persistence in the male reproductive tract.
To investigate the consequences of sexual transmission of ZIKV to females and developing fetuses, researchers developed a vaginal ZIKV transmission model in mice (Yockey et al., 2016). Following intravaginal (ivag) inoculation of a Cambodian strain of ZIKV into WT C57BL/6 mice, new virus production was detected in vaginal washes, demonstrating that the female reproductive tract of immunocompetent mice supports robust ZIKV replication. Similar to other routes of inoculation, ivag inoculation of Ifnar1−/− mice with ZIKV resulted in a lethal infection with high viral loads detected in a variety of tissues. Although not lethal, ivag inoculation of Tlr7−/−;Mavs−/− mice or Irf3−/−;Irf7−/− mice resulted in elevated viral loads in the female reproductive tract (Yockey et al., 2016), indicating that innate immune pathways control ZIKV replication in this tissue. Furthermore, as discussed in the next section, this model also can be used as a system to investigate the impact of sexually transmitted ZIKV to the developing fetus. Finally, based on the observations that ZIKV infection of male Ifnar1−/− mice results in high levels of infection in the male reproductive tract (Ma et al., 2017; Uraki et al., 2017b), researchers tested the capacity of ZIKV-infected Ifnar1−/− male mice to transmit ZIKV to cohoused naïve Ifnar1−/− female mice (Uraki et al., 2017a). Although evidence of ZIKV infection in the cohoused Ifnar1−/− female mice could not be detected, some fetuses from Ifnar1−/− female mice cohoused with ZIKV-infected, but not uninfected, male mice displayed IUGR, ocular malformations, and virions in the brain (detected by transmission electron microscopy) (Uraki et al., 2017a).
Animal Models of ZIKV Infection During Pregnancy
ZIKV infection of pregnant women is a major public health concern. ZIKV infection during pregnancy is associated with fetal microcephaly and other fetal disorders such as placental insufficiency, fetal growth restriction, ocular disorders, and fetal demise (Brasil et al., 2016; Cauchemez et al., 2016; Franca et al., 2016; Johansson et al., 2016; Oliveira Melo et al., 2016; Rasmussen et al., 2016; Sarno et al., 2016; Schuler-Faccini et al., 2016; Ventura et al., 2016a,b). To date, 16 countries and territories in the Americas have reported confirmed cases of ZIKV congenital syndrome, with the bulk of these occurring in Brazil. Accordingly, efforts have been made to develop experimental model systems of ZIKV pathogenesis in developing fetuses. Thus far, ZIKV infection of pregnant mice and NHPs has been reported to result in pathologic changes in fetuses (Adams Waldorf et al., 2016; Mysorekar and Diamond, 2016). For experiments in mice, ZIKV was inoculated into pregnant mice or the virus was inoculated directly into the brain of the developing fetus. These models employed WT mice and mice with genetic deficiencies in innate antiviral responses, such as Ifnar1−/− mice. Infection of pregnant Ifnar1−/− C57BL/6 mice with a contemporary Asian lineage ZIKV strain (H/PF/2013), via subcutaneous inoculation in the footpad at embryonic days 6.5 (E6.5) and E7.5, led to placental infection, fetal resorption, and fetal brain injury with accompanying neural cell death (Miner et al., 2016a). For these experiments, Ifnar1−/− female mice were bred with WT males resulting in fetuses that were heterozygous for Ifnar1. Thus, despite the fetuses having the ability to respond to type I IFN, at least partially, severe fetal outcomes were observed, suggesting that an intact type I IFN response in the fetus is not sufficient to protect from ZIKV-induced injury. In parallel to these experiments, pregnant WT C57BL/6 mice were treated with anti-IFNAR1 antibody 1–2 days prior to ZIKV inoculation on E6.5 or E7.5. Fetuses from mice treated with anti-IFNAR1 displayed intrauterine growth restriction (IUGR) and ZIKV infection. In both model systems, ZIKV infection was detected in fetal heads. This study also reported detection of ZIKV infection in cells lining the maternal-fetal interface (Miner et al., 2016a), suggesting that this model can be exploited not only to define mechanisms of ZIKV-induced fetal demise and IUGR, but also to define mechanisms of transplacental transmission of ZIKV to the fetus. In additional studies performed in C57BL/6 mice, researchers found that ivag inoculation of WT C57BL/6 mice with a Cambodian strain of ZIKV (FSS13025) results in a mild reduction in fetal weight and viral antigen in fetal brain (Yockey et al., 2016). Similar to other systems, vaginal infection of pregnant Ifnar1−/− mice resulted in more severe consequences for the fetus, further demonstrating that this model can be used to investigate ZIKV pathogenesis in the developing fetus.
In contrast to intravenous and subcutaneous inoculation of ZIKV into pregnant WT C57BL/6 mice, intravenous inoculation of pregnant WT SJL mice with a Brazilian strain of ZIKV was found to cause IUGR of developing fetuses, cortical malformations and a reduction of cortical neurons in fetal brains, and fetal ocular abnormalities (Cugola et al., 2016). These effects were associated with the presence of ZIKV RNA in the brain of the fetuses. Although this model system seems to require inoculation of extraordinarily high doses of ZIKV (4 × 1010 PFU) via an intravenous route, this system may have utility for investigating mechanisms of ZIKV teratogenicity in WT immunocompetent animals.
The effects of ZIKV infection on the fetus also have been evaluated in pregnant Stat2-deficient Syrian golden hamsters (Siddharthan et al., 2017). Following subcutaneous inoculation of the dam at day 8.5 with ZIKV, and evaluation of infection levels 6 days later, ZIKV was detected in placental tissues and fetal brains. However, under these experimental conditions, IUGR was not observed (Siddharthan et al., 2017).
Efforts also have been made to develop an experimental model of ZIKV teratogenicity in NHPs that more closely resemble features of human pregnancy. In one study, subcutaneous inoculation of a pregnant pigtail macaque with a Cambodian ZIKV strain at a time point corresponding to ~ 28 weeks of human pregnancy resulted in injury to the fetal brain characterized by reduced growth of the fetal biparietal diameter (the diameter across the fetal skull), white matter deficiency, white matter gliosis, and axonal damage (Adams Waldorf et al., 2016). ZIKV RNA was detected in the chorionic villous tissue of the placenta as well as the fetal brain and liver, suggesting transplacental transmission of ZIKV to the fetus followed by viral invasion of the fetal CNS and brain injury. Although infection of this pregnant pigtail macaque did not result in clinically apparent infection of the mother, ZIKV RNA was detected in the maternal brain, eyes, spleen, and liver. This initial study suggests that pregnant pigtail macaques can serve as an experimental animal model to investigate ZIKV pathogenesis in the developing fetus. Maternal-fetal ZIKV transmission also has been evaluated in rhesus macaques (Nguyen et al., 2017). In this study, pregnant macaques were inoculated subcutaneously with a Polynesian ZIKV strain either during the mid-first trimester (31 or 38 days’ gestation) or during the late second/early third trimester (103 or 118 days’ gestation). Although assessment of fetal growth by sonography did not reveal major fetal or placental abnormalities, fetal head circumference was reduced during the last month of pregnancy. Analysis of fetuses at 153–158 days of gestation following surgical delivery also did not reveal gross abnormalities; however, ZIKV RNA was detected in numerous fetal tissues. Notably, fetuses from animals infected during the first trimester had ocular pathology including inflammation of the retina and optic nerve (Nguyen et al., 2017).
Other models, both mammalian and nonmammalian, have been developed to assess the impact of ZIKV infection on the fetus. In contrast to the experimental model systems discussed, these approaches do not model transplacental transmission. Instead, ZIKV was injected directly into the developing fetus or embryo. For example, injection of ZIKV (Asian lineage strain SZ01) into the cerebroventricular space of fetuses developing in ICR or WT C57BL/6 mice at E13.5 resulted in decreased brain size, thinning of cortical layers, reduced numbers of cortical neural progenitors, and death of immature and mature neurons within 3–5 days postinfection (Li et al., 2016a; Wu et al., 2016). In a similar type of approach, one group evaluated the utility of chick embryos as an experimental model system to study ZIKV pathogenesis (Goodfellow et al., 2016). In these studies, different doses of ZIKV (strain MEX1-44; isolated from mosquitoes captured in Mexico in 2016) were injected through the amniotic membrane on E2.5 or E5. Injected embryos became actively infected with ZIKV and the researchers observed virus dose-dependent mortality by day 3 postinfection. Inoculation of embryos at a later stage of development, E13, resulted in active ZIKV infection but no mortality. Magnetic resonance imaging (MRI) of ZIKV-infected chick embryos at E15 and E20 revealed brain malformation and reduced brain growth, particularly in the cortical regions, that was associated with increased ventricular volumes. Thus, experimental infection of chick embryos may provide a more high-throughput system to investigate mechanisms of ZIKV pathogenesis in developing embryos.
Summary
In summary, robust animal models that mimic various aspects of CHIKV and ZIKV infection in humans have been developed in rodents, NHPs, and other species. Each of these systems has specific advantages and limitations. Nevertheless, the development of these models has led to new knowledge regarding the pathogenesis of both CHIKV and ZIKV. Furthermore, these model systems serve as valuable resources for the evaluation of candidate vaccines and therapeutics against CHIKV and ZIKV infection. For example, numerous candidate CHIKV vaccines, including candidates that have now reached human trials (Chang et al., 2014; Ramsauer et al., 2015), have been evaluated for safety and for efficacy in the mouse and NHP models described in this chapter (Wang et al., 2008, 2011; Akahata et al., 2010; Mallilankaraman et al., 2011; Brandler et al., 2013; Chu et al., 2013; Hallengard et al., 2014a,b; Roy et al., 2014). Similarly, despite their recent development, the animal models described here have already been exploited for the evaluation of novel ZIKV vaccines (Abbink et al., 2016; Dowd et al., 2016; Larocca et al., 2016). Furthermore, these animal models are being used to test novel therapeutics, including monoclonal antibodies and small molecules, for protective efficacy against CHIKV and ZIKV disease (Couderc et al., 2009; Rulli et al., 2011; Fric et al., 2013; Goh et al., 2013; Hawman et al., 2013; Pal et al., 2013; Dagley et al., 2014; Chen et al., 2015; Sali et al., 2015; Julander et al., 2016; Karlas et al., 2016; Sapparapu et al., 2016; Stettler et al., 2016; Swanstrom et al., 2016; Varghese et al., 2016; Zhao et al., 2016). For example, treatment of pregnant mice with ZIKV-specific neutralizing human antibodies reduced placental and fetal infection (Sapparapu et al., 2016), suggesting that monoclonal antibody-based therapies and vaccines that elicit neutralizing antibody responses can protect against the devastating consequences of maternal-fetal ZIKV transmission that occurs following ZIKV infection of pregnant women. The continued development and characterization of animal models, including models that may better reflect the influence of host genetic variation, immune status, and comorbidities, on the diverse clinical manifestations of CHIKV and ZIKV infection remains an important priority.