In addition to the integument, the immune system plays a key role in the destruction of internal pathogens. The body can distinguish between self and nonself and can recognize and remember nonself qualities in other cells (antigens). This allows the body to recognize pathogens it has previously encountered and to mount a quicker immune response against these antigens if exposed to them again. The immune system has two major types of immunity: humoral, which involves antibody production, and cell-mediated, which involves cells that combat fungal and viral infections.
Another nonspecific defense mechanism employed by the immune system is the inflammatory response. When white blood cells are activated, they release chemicals, such as histamine, that activate the immune response. This response dilates and increases the permeability of blood vessels. These effects together increase the flow of white blood cells and other immune cells to the affected area, allowing the body to more effectively ward off infection. Inflammation is often accompanied by the rise in body temperature termed a fever, which in theory increases the ability to fight infection by killing temperature-dependent pathogens and speeding up healing processes. However, whether or not fever is practically beneficial is still a topic of scientific debate.
The lymphatic system is another important part of the immune system and is found in the extravascular space of most tissues. Lymph flows through the lymphatic vessels from lymph node to lymph node. The lymph nodes and spleen serve as reservoirs of white blood cells and filters for lymph, removing antigen-presenting cells and foreign matter and activating the immune system when necessary (see Chapter 17, Circulatory and Respiratory Systems).
The immune system contains several varieties of white blood cells, or leukocytes, each with a specific function.

Granulocytes are attracted to the site of injury, where they phagocytize antigens and antigenic material.
Neutrophils, the most common type of granulocyte, are often the first responders to sites of inflammation. These cells are attracted to cytokines and in turn attract additional white blood cells once they arrive at the site of tissue damage. Although they can help moderate various infections and environmental trauma, neutrophils are particularly adapted to attack bacteria. Neutrophil counts are often elevated during the acute stages of inflammation and are the main component of pus.
Eosinophils are much less common and are responsible for immune responses, especially allergic and asthmatic responses. Elevated eosinophil counts on a complete blood count (CBC) often indicate an allergic response or infection by a parasite, including those that live on the surface of the skin (ectoparasites), such as fleas and ticks, and those that live in intercellular spaces (endoparasites), such as the parasitic worms known as helminths.
Basophils and the related mast cells are similarly involved in allergic responses and parasite infections and often are responsible for the release of histamine, which stimulates blood vessel dilation as previously described.
Monocytes are large, long-lived immune cells that can differentiate into macrophages and dendritic cells.
The main role of macrophages is to phagocytize dead cells and pathogens. If a pathogen is ingested, its antigens are then presented on the surface of the macrophage to stimulate other immune cells to mount a specific immune response to the invading pathogen.
Dendritic cells are even more focused on processing antigens and presenting them to other immune cells and therefore serve as important links between the innate and adaptive immune systems. Dendritic cells are found in areas of the body where contact with the external environment is more common (e.g., the skin, intestine, and mucous membranes).
T cells are an important component in specific immunity. Through rearrangement of the chains that compose its antigen receptors, each T cell becomes reactive to only one random antigen type, usually as presented by a major histocompatibility protein complex (MHC). The vast majority of T cells created are subsequently deactivated and undergo apoptosis because they either will not react with the MHC or because they react too well and would attack self cells. Nevertheless, although each T cell can only respond to one specific antigen type and many are destroyed during development, a sufficient number of T cells to defend the body against nearly any pathogen are created and allowed to circulate.
The two major types of histocompatibility proteins are MHC I and MHC II. Cytotoxic T (TC) cells (also known as CD8+ T cells because they contain the CD8 protein) recognize and respond to antigens presented by MHC I complexes. These complexes come from cells infected with viruses or developing tumors and signal TC cells to destroy those cells. In contrast, T helper (TH) cells (also known as CD4+ T cells) recognize and respond to antigens presented by MHC II complexes. Activated TH cells release cytokines to stimulate the immune response, causing other white blood cells to mature and attack. Natural killer T (NKT) cells behave similarly to both TC and TH cells but respond to antigens presented by other types of cells.
Once a reaction has occurred, memory T cells reactive to the same antigens are formed and remain in circulation for long periods of time, allowing a quicker, more targeted response if the antigen reappears. Regulatory or suppressor T (Treg) cells have the opposite function, serving to tone down T cell response to self cells or following an infection.
T cells begin their development in the bone marrow, where T lymphocyte precursor cells are formed. They travel via the bloodstream to the thymus, where they mature. It is because these cells mature in the thymus that they are referred to as T cells. Once maturation is complete, these cells are released into the lymph to perform their immune function.
T cells are a vital component of the immune system. Patients with acquired immunodeficiency syndrome (AIDS) have very low levels of certain types of T cells and as a result are particularly subject to infection because the immune system is so weakened by this loss.
B cells, when stimulated, create and express antibodies (also known as immunoglobulins) that have a high affinity for the antigen expressed by the stimulating T lymphocyte. Immunoglobulins have a very particular structure (outlined later in this chapter) and utilize the specificity of this structure to aid in the targeted destruction of pathogens. Like T cells, B cells can also stimulate the formation of memory cells.
B lymphocytes, like T cells, begin their development in the bone marrow. However, unlike T cells, their development is completed there; they do not travel to other parts of the body to mature.
Table 14.1 below outlines the most important cells found in the immune system as well as their functions.
| Cell Type | Function |
| Granulocytes | |
| Basophil | Least common of all the granulocytes (1%); fight parasites; mediate allergic response |
| Eosinophil | Much less common than neutrophils (5%); fight parasites; mediate allergic response |
| Neutrophils | Most common of the granulocytes (94%); phagocytic |
| Monocytes | |
| Macrophage | Phagocytic; secrete cytokines; present antigens |
| Dendritic cells | Present antigens; activate immune system |
| Lymphocytes | |
| B cells | Produce antigen-specific antibodies |
| T cells | Helper T (CD4+) cells activate other immune cells; cytotoxic T (CD8+) cells and natural killer T (NKT) cells destroy cells marked for destruction; memory T cells remain after an infection so a response can be mounted more quickly if infected again |
The immune system also contains components that are not cells. Various chemicals, hormones, and enzymes supplement the action of the cells and serve equally important roles.
Large proteins secreted by B cells known as antibodies or immunoglobulins provide specific, targeted responses to a given antigen. Several types of immunoglobulins exist within the immune system, and each plays a unique role in immunity. Nevertheless, the structure of all immunoglobulins is relatively consistent and resembles a “Y,” with antigen binding sites at either end of the top of the “Y.” Each side of the structure consists of two chains, a light chain and a heavy chain, which are held together with disulfide bonds. The variable portion of the structure is the antigen-binding region.
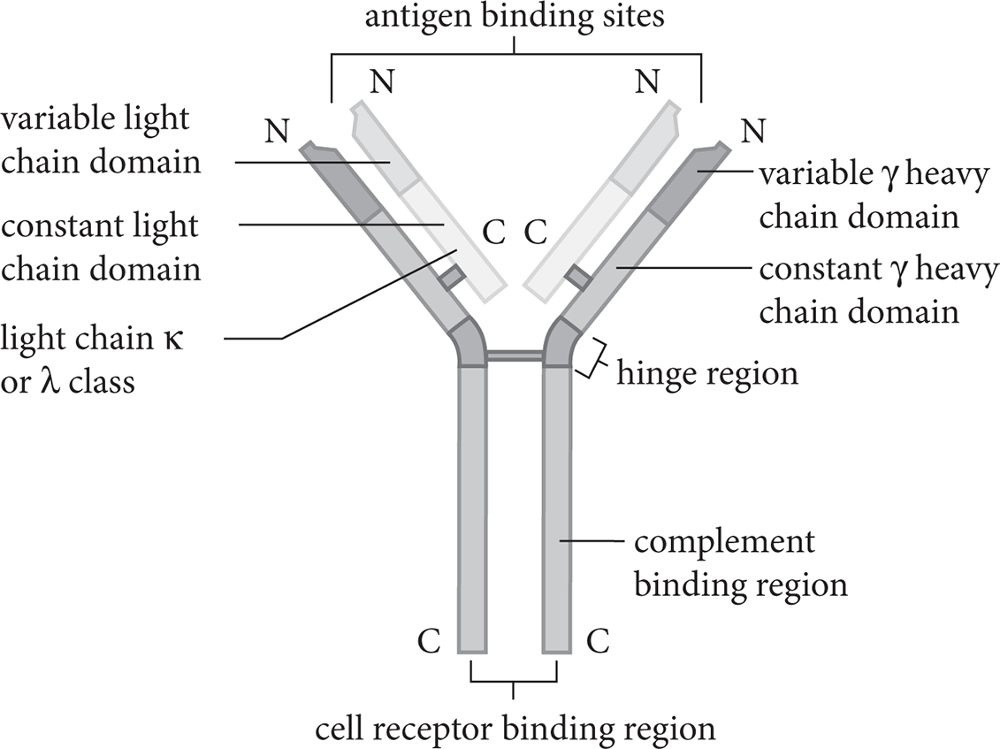
The antigen-binding region of the antibody is unique to each antigen and is the reason that specificity for a particular antigen exists. The immune system can generate millions of unique antigen binding sites, which confers the ability to mount an immune response against any number of antigens.
Antibody-mediated immunity includes both active and passive immunity. Active immunity occurs as a result of an immune response. This can be due to exposure to a pathogen or antigen, such as during an infection. It can also be the result of vaccination, where an individual is deliberately exposed to a weakened, inactivated, or killed form of the antigen. This exposure stimulates the body’s immune system to mount an immune response against the antigen presented. The features of this antigen are then stored in the “nonself” memory, allowing the body to mount a similar immune response should the antigen present itself again. This type of immune response, because it requires the development of cells specific to a particular antigen, can take weeks or months to build up.
Passive immunity, in contrast, is acquired by the transfer of antibodies from one individual to another. This can occur during pregnancy, for example, when maternal antibodies cross the placenta and enter fetal circulation. Injections of gamma globulin, which is the fraction of the blood containing antibodies, can also provide passive immunity by transferring antibodies to a particular illness to a given individual. While passive immunity is effective immediately upon transfer of antibodies, once the antibodies are no longer circulating in the immune system the effect of the immunity is lost.
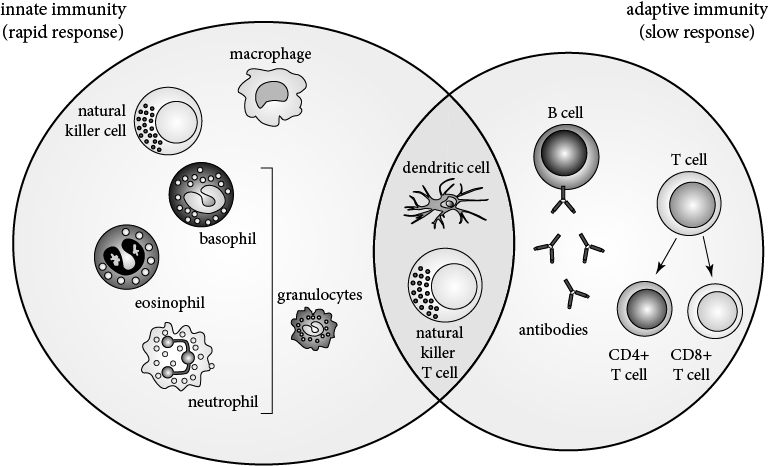
While immunity can be characterized by the types of immune components involved, it can also be divided into innate and adaptive immunity based on how the immunity is acquired. Each of these categories involves components from both cell-mediated and humoral immunity.
Innate immunity is comprised of the body’s initial, generalized defenses against pathogens. This type of immunity does not require the cells of the immune system to be previously exposed to any given antigen to be activated. However, it is not a specific response, and the body is limited in the types of immune response it can mount. Innate immunity includes:
Acquiredor specific immunity consists of cells capable of recognizing self versus nonself cells—for example, cells that can differentiate invading cells from host cells—and that are specific to a particular antigen. The activity of cells that participate in an adaptive immune response is increased with each exposure. Thus, there are memory components to acquired immunity: Cells recognize antigens they have been exposed to previously, and the immune response mounted against increases in magnitude after each repeat exposure to an antigen. Cells that are involved in adaptive immunity are:
Innate and acquired immunity work together to protect the host and defend against invading pathogens. Phagocytic cells can stimulate production of specific T lymphocytes to assist in pathogen killing and destruction. T lymphocytes, in turn, can release cytokines, which increase the killing activities of phagocytes. Other examples of this type of cooperation exist, and they work to increase the function and efficacy of the immune system.

Transplanted tissues or organs are detected as nonself by the recipient’s immune system. This is because the antigens on the donated organ are those of the donor, not of the recipient. As a result, the recipient’s immune system attacks the transplanted organ. This attack can lead to rejection of the organ, which can ultimately result in destruction of the organ or death of the patient. As a result, immunosuppressing drugs are used to lower the immune response to transplants and decrease the likelihood of rejection. These work by lowering the body’s immune response to antigens; while this decreases the likelihood of rejecting the organ, it also lowers the recipient’s overall immune response. The recipient is then referred to as immunocompromised because his or her immune system is not functioning at its full capacity.