|
When two or more atoms with similar electronegativities interact, the energy required to form ions is greater than the energy that would be released upon the formation of an ionic bond (i.e., the process is not energetically favorable). Since a complete transfer of electrons cannot occur, such atoms achieve a noble gas electron configuration by sharing electrons in a covalent bond. The binding force between the two atoms results from the attraction that each electron of the shared pair has for the two positive nuclei.
Covalent compounds generally contain discrete molecular units with weak intermolecular forces. Consequently, they have low melting points and do not conduct electricity in the liquid or aqueous states.
Atoms can share more than one pair of electrons. Two atoms sharing one, two, or three electron pairs are said to be joined by a single, double, or triple covalent bond, respectively. The number of shared electron pairs between two atoms is called the bond order; hence, a single bond has a bond order of one, a double bond has a bond order of two, and a triple bond has a bond order of three.
Two key features characterize covalent bonds: bond length and bond energy.
Bond length is the average distance between the two nuclei of the atoms involved in the bond. As the number of shared electron pairs increases, the two atoms are pulled closer together, leading to a decrease in bond length. Thus, for a given pair of atoms, a triple bond is shorter than a double bond, which is shorter than a single bond.
Bond energy is the energy required to separate two bonded atoms. For a given pair of atoms, the strength of a bond (and therefore the bond energy) increases as the number of shared electron pairs increases.
The shared valence electrons of a covalent bond are called the bonding electrons. The valence electrons not involved in the covalent bond are called nonbonding electrons. These unshared electron pairs can also be called lone electron pairs. A convenient notation, called a Lewis structure, is used to represent the bonding and nonbonding electrons in a molecule, facilitating chemical “bookkeeping.” The number of valence electrons attributed to a particular atom in the Lewis structure of a molecule is not necessarily the same as the number would be in the isolated atom, and the difference accounts for what is referred to as the formal charge of that atom. Often, more than one Lewis structure can be drawn for a molecule; this phenomenon is called resonance. Lewis structures, formal charge, and resonance are discussed in detail below.
When different atoms interact to form a bond, only their outermost regions come in contact. Hence, only the valence electrons are involved. One of the easiest ways to follow the valence electrons in a chemical reaction is with Lewis dot symbols. A Lewis dot symbol contains the symbol of an element and one “dot” for each valence electron in an atom. Magnesium, for example, belongs to Group IIA and has two valence electrons (:Mg). Note: Because the transitional-metal lanthanide and actinides all have incompletely filled inner shells, Lewis dot symbols are not written for these elements.
|
Just as a Lewis symbol is used to represent the distribution of valence electrons
in an atom, one can also be used to represent the distribution of valence electrons
in a molecule. For example, the Lewis symbol of an F ion is ![]() the Lewis structure of an F2 molecule is
the Lewis structure of an F2 molecule is ![]()
Certain steps must be followed in assigning a Lewis structure to a molecule. These steps are outlined below, using hydrogen cyanide (HCN) as an example.
1. Count all the valence electrons of the atoms. The number of valence electrons of the molecule is the sum of the valence electrons of all atoms present:
2. Write the skeletal structure of the compound (i.e., the arrangement of atoms). In general, the least electronegative atom is the central atom. Hydrogen (always) and the halogens F, Cl, Br, and I (usually) occupy the end positions. Draw single bonds between the central atom and the atoms surrounding it, placing an electron pair in each bond (bonding electron pair).
In HCN, H must occupy a terminal position. Of the remaining two atoms, C is the less electronegative and therefore occupies the central position. The skeletal structure is as follows:
Each bond has 2 electrons, so 10 − 4 = 6 valence electrons remain.
3. Complete the octets (8 valence electrons) of all atoms bonded to the central atom, using the remaining valence electrons still to be assigned. (Recall that H is an exception to the octet rule since it can have only 2 valence electrons.) In this example H already has 2 valence electrons in its bond with C.

4. Place any extra electrons on the central atom. If the central atom has less than an octet, try to write double or triple bonds between the central and surrounding atoms using the nonbonding, unshared lone electron pairs. The HCN structure above does not satisfy the octet rule for C because C possesses only 4 valence electrons. Therefore, 2 lone electron pairs from the N atom must be moved to form two more bonds with C, creating a triple bond between C and N.
5. Finally, bonds are drawn as lines rather than pairs of dots.
Now, the octet rule is satisfied for all three atoms because C and N have 8 valence electrons and H has 2 valence electrons.
The number of electrons officially assigned to an atom in a Lewis structure does not always equal the number of valence electrons of the free atom. The difference between these two numbers is the formal charge of the atom. Formal charge can be calculated using the following formula:

where V is the number of valence electrons in the free atom, Nbonding is the number of bonding electrons, and Nnonbonding is the number of nonbonding electrons. Using a Lewis dot structure, where 2 bonding electrons are represented by a stick and each nonbonding electron is represented with a dot, the formal charge is also represented by:
The formal charge of an ion or molecule is equal to the sum of the formal charges of the individual atoms comprising it.
Example:
Calculate the formal charge on the central N atom of [NH4]+.
Solution:
The Lewis structure of [NH4]+ is

Nitrogen is in Group VA; thus, it has 5 valence electrons. In [NH4]+, N has 4 bonds (i.e., 8 bonding electrons and no nonbonding electrons).
So V = 5; Nbonding = 8; Nnonbonding = 0

Likewise, there are 4 “sticks” and 0 “dots” in the Lewis structure, so formal charge (FC) could be calculated like this too:

Thus, the formal charge on the N atom in [NH4]+ is +1.
For some molecules, two or more non-identical Lewis structures can be drawn; these arrangements are called resonance structures. A resonance structure is one of two or more Lewis structures for a single molecule unable to be described fully with only one Lewis structure. The molecule doesn’t actually exist as either one of the resonance structures but is rather a composite, or hybrid, of the two.
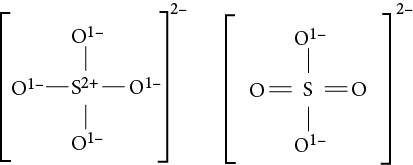
For example, SO2 has three resonance structures, two of which are minor: O=S−O and O−S=O. The actual molecule is a hybrid of these three structures (spectral data indicate that the two S−O bonds are identical). This phenomenon is known as resonance, and the actual structure of the molecule is called the resonance hybrid. Resonance structures are expressed with a double-headed arrow between them:

The last two resonance structures of sulfur dioxide (shown above) have equivalent energy or stability. Often, nonequivalent resonance structures may be written for a molecule. In these cases, the more stable the structure, the more that structure contributes to the character of the resonance hybrid. Conversely, the less stable the resonance structure, the less that structure contributes to the resonance hybrid. The structure on the left of the diagram is the most stable. In this case, the most stable structure has no formal charge on any of the component atoms. Sulfur has more than 8 valence electrons (10 in this case), but that is acceptable because sulfur has a d subshell that can serve to expand the octet. More exceptions to the octet rule are discussed below.
Therefore, formal charges are often useful for qualitatively assessing the stability of a particular resonance structure, and the following guidelines are used.
Example:
Write the resonance structures for the cyanate anion (which contains one nitrogen atom, one oxygen atom, one carbon atom and an overall charge of negative one).
Solution:
First, tally the total number of valence electrons:
N has 5 valence electrons;
C has 4 valence electrons;
O has 6 valence electrons; and the species itself has one negative charge (meaning one additional electron).
Total valence electrons = 5 + 4 + 6 + 1 = 16
C is the least electronegative of the three given atoms, N, C, and O. Therefore, the C atom occupies the central position in the skeletal structure of [NCO]−. Draw single bonds between the central C atom and the surrounding atoms, N and O, and place a pair of electrons in each bond.
Complete the octets of N and O with the remaining (16 – 4) = 12 electrons.

The C octet is incomplete. There are three ways in which double and triple bonds can be formed to complete the C octet. Two lone pairs from the O atom can be used to form a triple bond between the C and O atoms:

Or one lone electron pair can be taken from both the O and the N atoms to form two double bonds, one between N and C and the other between O and C:

Or two lone electron pairs can be taken from the N atom to form a triple bond between the C and N atoms:

These three are all resonance structures of [NCO]−.
Assign formal charges to each atom of each resonance structure: In the first structure, the N has a formal charge of −2, and the O has a charge of +1. In the second structure the N has a formal charge of −1, and in the third structure the O has a charge of −1.
The most stable structure is therefore:

since the negative formal charge is on the most electronegative atom, O.
Atoms found in or beyond the third period can have more than eight valence electrons, since some of the valence electrons may occupy d orbitals. These atoms can be assigned more than four bonds in Lewis structures.
The Lewis structure of the sulfate ion,
 for example, can be drawn in six resonance forms by alternating the placement of pi
electrons. Giving the sulfur 12 valence electrons permits three of the five atoms
to be assigned a formal charge of zero, which is most favorable energetically.
for example, can be drawn in six resonance forms by alternating the placement of pi
electrons. Giving the sulfur 12 valence electrons permits three of the five atoms
to be assigned a formal charge of zero, which is most favorable energetically.
The nature of a covalent bond depends on the relative electronegativities of the atoms sharing the electron pairs. Covalent bonds are considered to be polar or nonpolar depending on the difference in electronegativities between the atoms.
Polar covalent bonding occurs between atoms with small differences in electronegativity, generally in the range of 0.4 to 1.7 Pauling units. The bonding electron pair is not shared equally but is pulled more toward the element with the higher electronegativity. As a result, the more electronegative atom acquires a partial negative charge, δ-, and the less electronegative atom acquires a partial positive charge, δ+, giving the molecule a partially ionic character. For instance, the covalent bond in HCl is polar because the two atoms have a small difference in electronegativity (approx. 0.9). Chlorine, the more electronegative atom, attains a partial negative charge, and hydrogen attains a partial positive charge. This difference in charge between the atoms is indicated by an arrow crossed (like a plus sign) at the positive end and pointing to the negative end, as shown below.

A molecule that has such a separation of positive and negative charges is called a polar molecule. The dipole moment itself is a vector quantity μ, measured in Debye units (coulomb-meters) and defined as the product of the charge magnitude (q) and the distance between the two partial charges (r):
Nonpolar covalent bonding occurs between atoms that have the same electronegativities. The bonding electron pair is shared equally such that there is no separation of charge across the bond. Nonpolar covalent bonds occur in diatomic molecules such as H2, Cl2, O2, and N2.
In a coordinate covalent bond, the shared electron pair comes from the lone pair of one of the atoms in the molecule. Once such a bond forms, it is indistinguishable from any other covalent bond, so identifying such a bond is useful only in keeping track of the valence electrons and formal charges.
Coordinate bonds are typically found in Lewis acid-base compounds (see Chapter 34, Acids and Bases). A Lewis acid is a compound that can accept an electron pair to form a covalent bond; a Lewis base is a compound that can donate an electron pair to form a covalent bond. For example, in the reaction between boron trifluoride (BF3) and ammonia (NH3):
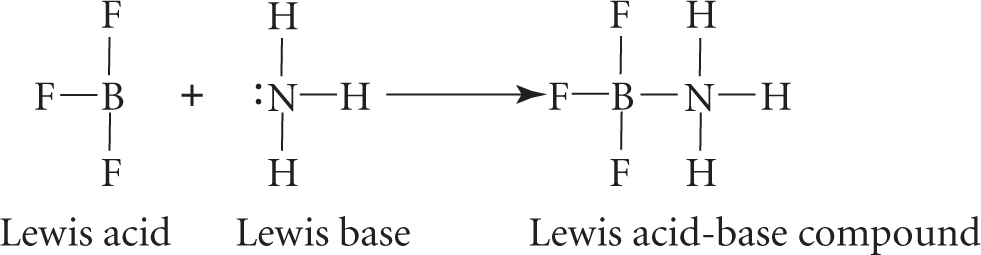
NH3 donates a pair of electrons to form a coordinate covalent bond; thus, it acts as a Lewis base. BF3 accepts this pair of electrons to form the coordinate covalent bond; thus, it acts as a Lewis acid.
The valence shell electron-pair repulsion (VSEPR) theory uses Lewis structures to predict the molecular geometry of covalently bonded molecules. It states that the three-dimensional arrangement of atoms surrounding a central atom is determined by the repulsions between the bonding and the nonbonding electron pairs in the valence shell of the central atom. These electron pairs arrange themselves as far apart as possible, thereby minimizing repulsion.
The following steps are used to predict the geometric structure of a molecule using the VSEPR theory:
For example, the compound AX2 has the Lewis structure (X:A:X). A has two bonding electron pairs in its valence shell. To make these electron pairs as far apart as possible, their geometric structure should be linear:
Valence electron arrangements are summarized in Table 26.1.
| Electron Pairs | Nonbonding Pairs | Example | Geometric Arrangement | Shape | Angles |
|---|---|---|---|---|---|
| 2 | 0 | BeCl2 |
 |
Linear | 180° |
| 3 | 0 | BH3 |
 |
Trigonal Planar | 120° |
| 4 | 0 | CH4 |
 |
Tetrahedral | 109.5° |
| 4 | 1 | NH3 |
 |
Trigonal Pyramidal | 107° |
| 4 | 2 | H2O |
 |
Bent | 104.5° |
| 5 | 0 | PCl5 |
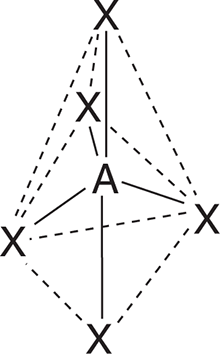 |
Trigonal Bipyramidal | 90°, 120°, 180° |
| 6 | 0 | SF6 |
 |
Octahedral | 90°, 180° |
Example:
Predict the geometry of NH3.
Solution:
The Lewis structure of NH3 is:

The central atom, N, has three bonding electron pairs and one nonbonding electron pair for a total of four electron pairs.
The four electron pairs will be farthest apart when they occupy the corners of a tetrahedron. Since one of the four electron pairs is a lone pair, the observed geometry is trigonal pyramidal.

In describing the shape of a molecule, only the arrangement of atoms (not electrons) is considered. Even though the electron pairs are arranged in a tetrahedron, the shape of NH3 is pyramidal. It is not trigonal planar because the lone pair repels the three bonding electron pairs, causing them to move as far away as possible.
Example:
Predict the geometry of CO2.
Solution:
The Lewis structure of CO2 is 
The double bond behaves just like a single bond for purposes of predicting molecular shape. This compound has two groups of electrons around the carbon. According to the VSEPR theory, the two sets of electrons will orient themselves 180° apart, on opposite sides of the carbon atom, to minimize electron repulsion. Therefore, the molecular structure of CO2 is linear.
A molecule with a net dipole moment is called polar, as previously mentioned, because it has positive and negative poles. The polarity of a molecule depends on the polarity of the constituent bonds and on the shape of the molecule. A molecule with nonpolar bonds is always nonpolar; a molecule with polar bonds may be polar or nonpolar depending on the orientation of the bond dipoles.
A molecule of two atoms bound by a polar bond must have a net dipole moment and therefore be polar. The two equal and opposite partial charges are localized at the ends of the molecule on the two atoms. A molecule consisting of more than two atoms bound with polar bonds may be either polar or nonpolar since the overall dipole moment of a molecule is the vector sum of the individual bond dipole moments. If the molecule has a particular shape such that the bond dipole moments cancel each other (i.e., if the vector sum is zero), then the result is a nonpolar molecule. For instance, CCl4 has four polar C−Cl bonds. According to the VSEPR theory, the shape of CCl4 is tetrahedral. The four bond dipoles point to the vertices of the tetrahedron and cancel each other, resulting in a nonpolar molecule:
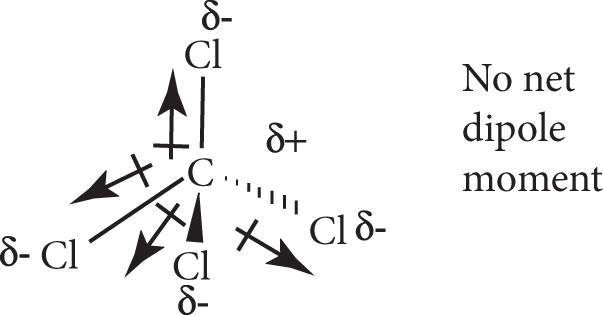
However, if the orientation of the bond dipoles are such that they do not cancel out, the molecules will have a net dipole moment and therefore be polar. For instance, H2O has two polar O−H bonds. According to the VSEPR model, its shape is angular. The two dipoles add together to give a net dipole moment to the molecule, making the H2O molecule polar:
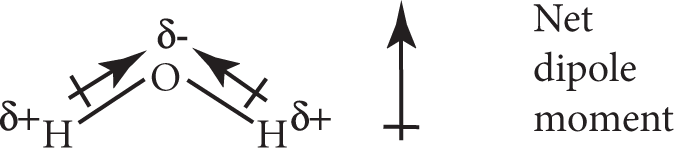
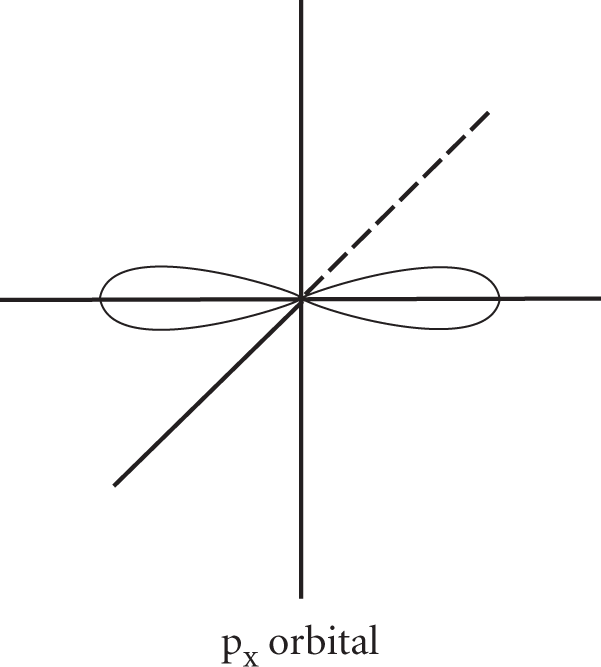
A description of the quantum numbers has already been given in Chapter 24, Atomic Structure. The azimuthal quantum number (ℓ) describes the orbitals of each n shell. The shapes of these orbitals represent the probability of finding an electron at any given instant. ℓ = 0 represents an s orbital, and s orbitals are spherically symmetric. The 1s orbital (n = 1, ℓ = 0) is plotted below.

When ℓ = 1 there are three possible orbitals (since the magnetic quantum number, mℓ, may equal −1, 0, or +1). These are called p orbitals and have dumbbell shapes. The three p orbitals, designated px, py, and pz are oriented at right angles to each other; the px orbital is plotted below.
The shapes of the five d orbitals (ℓ = 2, mℓ = −2, −1, 0, 1, 2) and the seven f orbitals (ℓ = 3, mℓ = −3, −2, −1, 0, 1, 2, 3) are more complex and need not be memorized for the PCAT.
When two atoms bond to form a molecule, the atomic orbitals interact to form a molecular orbital that describes the probability of finding the bonding electrons. Molecular orbitals are obtained by adding the wave functions of the atomic orbitals. Qualitatively, this is described by the overlap of two atomic orbitals. If the signs of the two atomic orbitals are the same, a bonding orbital is formed. If the signs are different, an antibonding orbital is formed. When two orbitals of different atoms overlap head-to-head, the resulting bond is called a sigma (σ ) bond. When parallel p orbitals interact, a pi (π ) bond is formed.