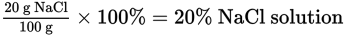
Concentration denotes the amount of solute dissolved in a solvent. The concentration of a solution is most commonly expressed as percent composition by mass, mole fraction (X), molarity (M), molality (m), or normality (N).
The percent composition by mass (%) of a solute is the mass of the solute divided by the mass of the solution (solute plus solvent) and multiplied by 100.
Example:
What is the percent composition by mass of NaCl of a saltwater solution if 100 g of the solution contains 20 g of NaCl?
Solution:
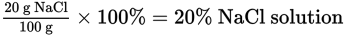
The mole fraction (X) of a compound is equal to the number of moles of the compound divided by the total number of moles of all species within the system. The sum of all the mole fractions in a system will always equal 1.
The mole fraction can be calculated with the following equation:
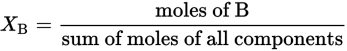
where XB is the mole fraction of component B.
Example:
If 92 g of glycerol is mixed with 90 g of water, what will be the mole fractions of the two components (the molecular mass of H2O = 18 g/mol and the molecular mass of C3H8O3 = 92 g/mol)?
Solution:
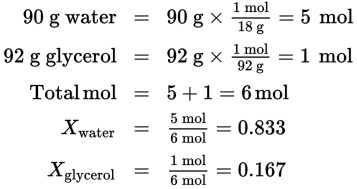
As a check, note that the sum of Xwater and Xglycerol is 1:
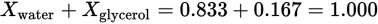
The molarity (M) of a solution is the number of moles of solute per liter of solution. Solution concentrations are usually expressed in terms of molarity. Molarity depends on the total volume of the solution, not on the volume of solvent used to prepare the solution.
Example:
If enough water is added to 11 g of CaCl2 to make 100 mL of solution, what is the molarity of the solution (the molecular mass of CaCl2 is 110 g/mol)?
Solution:

The molality (m) of a solution is the number of moles of solute per kilogram of solvent. For dilute aqueous solutions at 25°C, the molality is approximately equal to the molarity because the density of water at this temperature is 1 kilogram per liter, but note that this is an approximation and true only for dilute aqueous solutions.
Example:
If 10 g of NaOH are dissolved in 500 g of water, what is the molality of the solution (the molecular mass of NaOH is 40 g/mol)?
Solution:
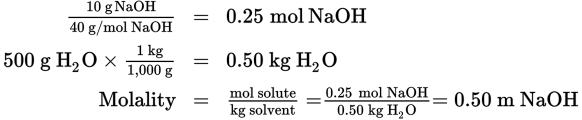
The normality (N) of a solution is equal to the number of gram equivalent weights of solute per liter of solution. A gram equivalent weight, or equivalent, is a measure of the reactive capacity of a molecule (see Chapter 27, Stoichiometry).
To calculate the normality of a solution, we must know for what purpose the solution is being used because it is the concentration of the reactive species with which we are concerned. Normality is unique among concentration units in that it is reaction dependent. For example, each mole of sulfuric acid contributes 2 equivalents for acid-base reactions (because each mole of sulfuric acid provides 2 moles of H+ ions) but contributes only 1 equivalent for a sulfate precipitation reaction (because each mole of sulfuric acid provides only 1 mole of sulfate ions). You can calculate normality by multiplying the molarity (M) of a solution by the number of equivalents per mol:
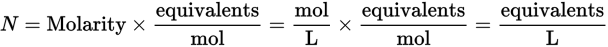
Therefore, a 3 M solution of H2SO4 would be 6 N in acid-base reactions (3 M × 2 equivalents/mol = 6 N) and 3 N in sulfate precipitation reactions (3 M × 1 equivalents/mol = 3 N).
A solution is diluted when solvent is added to a solution of higher concentration to produce a solution of lower concentration. The concentration of a solution after dilution can be conveniently determined using the equation below:
where M is molarity, V is volume, and the subscripts i and f refer to initial and final values, respectively.
Example:
How many mL of a 5.5 M NaOH solution must be used to prepare 300 mL of a 1.2 M NaOH solution?
Solution:

The solubility of a substance varies depending on the temperature of the solution, the solvent, and, in the case of a gas-phase solute, the pressure. Solubility is also affected by the addition of other substances to the solution.
The most common class of solutions is aqueous solutions (in which the solvent is water). The aqueous state is denoted by the symbol (aq). When discussing the chemistry of aqueous solutions and answering questions on the PCAT, it is useful to know how soluble various salts are in water; this information is given by the solubility rules below.
Soluble salts (with exceptions):
Insoluble salts (with exceptions):