
Chapter Twenty-Nine
Elements and compounds can react to form other species in many ways, and memorizing every reaction would be impossible as well as unnecessary. However, nearly every inorganic reaction can be classified into at least one of five general categories.
Combination reactions are reactions in which two or more reactants form one product. The formation of sulfur dioxide by burning sulfur in air is an example of a combination reaction:
Combination reactions can also occur when two compounds react to form a new compound. For example, in the equation below, gaseous ammonia is reacted with gaseous hydrogen chloride and forms ammonium chloride:
A decomposition reaction is defined as one in which a compound breaks down into two or more substances, usually as a result of heating or electrolysis. For example, when compounds that contain oxygen are heated, most will decompose to form molecular oxygen. Electrolysis is a specific process that causes the decomposition of a compound by passing an electric current through the reactant. Another example of a decomposition reaction is the breakdown of mercury (II) oxide (the sign Δ represents the addition of heat):

Single displacement reactions occur when an atom (or ion) of one compound is replaced by an atom of another element. For example, zinc metal will displace copper ions in a copper sulfate solution to form zinc sulfate:
Single displacement reactions are often further classified as redox reactions, discussed below.
Because many reactions, including some displacements, involve ions in solution, their net equations can also be written in ionic form. In the example where zinc is reacted with copper sulfate, the ionic equation is:
When displacement reactions occur, there are usually spectator ions that do not take part in the overall reaction but simply remain in solution throughout. The spectator ion in the equation above is sulfate, which does not undergo any transformation during the reaction. A net ionic reaction can be written showing only the species that actually participate in the reaction:
Net ionic equations are important for demonstrating the actual reaction that occurs during a displacement reaction.
In double displacement reactions, also called metathesis reactions, elements from two different compounds displace each other to form two new compounds. This type of reaction occurs when one of the products is removed from the solution as a precipitate or gas, or when two of the original species combine to form a weak electrolyte that remains undissociated in solution. For example, when solutions of calcium chloride and silver nitrate are combined, insoluble silver chloride forms in a solution of calcium nitrate:
Neutralization reactions are a specific type of double displacement that occurs when an acid reacts with a base to produce a solution of a salt and water. For example, hydrochloric acid and sodium hydroxide will react to form sodium chloride and water.
This type of reaction will be discussed further in Chapter 34, Acids and Bases.
A reaction that involves the transfer of electrons from one species to another is an oxidation-reduction (redox) reaction. This type of reaction can be divided into two half reactions, oxidation (loss of electrons) and reduction (gain of electrons). The law of conservation of charge states that an electrical charge can be neither created nor destroyed. Thus, an isolated loss or gain of electrons cannot occur; oxidation and reduction must occur simultaneously, resulting in an electron transfer called a redox reaction. An oxidizing agent causes another atom in a redox reaction to undergo oxidation, and itself is reduced. A reducing agent causes the other atom to be reduced, and itself is oxidized.
It is important to know which atom is oxidized and which is reduced. Oxidation numbers are assigned to atoms to keep track of the redistribution of electrons during a chemical reaction. From the oxidation numbers of the reactants and products, it is possible to determine how many electrons are gained or lost by each atom. The oxidation number is the number of charges an atom would have in a molecule if electrons were completely transferred in the direction indicated by the difference in electronegativity. Along the same lines, an element is said to be oxidized (loses electrons) if its oxidation number increases in a given reaction, and an element is said to be reduced (gains electrons) if the oxidation number of the element decreases in a given reaction. The oxidation number of an atom in a compound is assigned according to the following rules:
Example:
Assign oxidation numbers to the atoms in the following reaction to determine the oxidized and reduced species and the oxidizing and reducing agents:
Solution:
All these species are neutral, so the oxidation numbers of each compound must add up to zero. In SnCl2, since there are two chlorines present and chlorine has an oxidation number of −1, Sn must have an oxidation number of +2. Similarly, the oxidation number of Sn in SnCl4 is +4; the oxidation number of Pb is +4 in PbCl4 and +2 in PbCl2. Notice that the oxidation number of Sn increases from +2 to +4; it loses electrons and thus is oxidized, making it the reducing agent. Since the oxidation number of Pb has decreased from +4 to +2, it has gained electrons and been reduced. Pb is the oxidizing agent. The sum of the charges on both sides of the reaction is equal to zero, so charge has been conserved.
Balancing any reaction requires not only stoichiometric balance but also charge balance. Because redox reactions involve the transfer of electrons (and therefore of charge) between different elements, balancing redox reactions introduces an additional level of complexity. However, with a straightforward, stepwise method, balancing chemical reactions on the PCAT becomes more manageable.
By assigning oxidation numbers to the reactants and products, one can determine how many moles of each species are required for conservation of charge and mass, which is necessary to balance the equation. To balance a redox reaction, both the net charge and the number of atoms must be equal on both sides of the equation. The most common method for balancing redox equations is the half-reaction method, also known as the ion-electron method, in which the equation is separated into two half reactions—the oxidation part and the reduction part. Each half reaction is balanced separately, and they are then added to give a balanced overall reaction.
Example:
Balance the redox reaction between MnO4 and I in an acidic solution:
Solution:
Note how the oxidation numbers for I and Mn change (I changes from an oxidation state of −1 to 0, and Mn changes from +7 to +2) and that the reaction is not balanced. So, use the 5-step method to balance redox reactions.
Step 1:
Separate the two half reactions.

Step 2:
Balance the atoms of each half reaction. First, balance all atoms except H and O. Next, add H2O to balance the O atoms and then add H+ to balance the H atoms.
To balance the iodine atoms, place a coefficient of two before the I− ion:
For the permanganate half reaction, Mn is already balanced. Balance the oxygen atoms by adding 4 H2O to the right side:
Finally, add H+ to the left side to balance the 4 H2Os. These two half reactions are now balanced:
Step 3:
Balance the electrons of each half reaction. Each half reaction must have the same net charge on the left and right sides, and the only species that can be used to balance charges are electrons, each with a −1 charge. Additionally, the reduction half reaction must consume the same number of electrons as supplied by the oxidation half.
For the oxidation reaction, the left side has a charge of −2, and the right side has a charge of 0. Add 2 electrons to the right side of the reaction to balance both sides to be −2:
For the reduction reaction, there is a charge of +7 on the left and +2 on the right. By adding 5 electrons to the left side, the charges on both sides become +2, balancing the half reaction:
Next, both half reactions must have the same number of electrons so that, when the half reactions are combined, the electrons cancel. Therefore, multiply the oxidation half by 5 and the reduction half by 2.
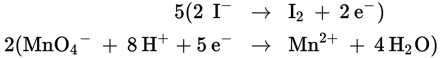
Step 4:
Combine the half reactions.

The sum of the two equations is:
To find the final equation, cancel out the electrons and anything that appears on both sides of the equation:
Step 5:
Confirm that mass and charge are balanced. Here, there is a +4 net charge on each side of the reaction equation, and the atoms are stoichiometrically balanced.
For redox reactions in a basic (instead of acidic) solution, an additional step is required because H+ will not be readily available and should not appear as a reactant. Use the same initial steps 1−4, but then add enough hydroxide (OH−) to both sides to completely combine with the free H+, forming water. Then, remove any species that appears on both sides and complete step 5 as usual to confirm everything is still balanced.
For example, in order to balance the same redox reaction of MnO4 and I in a basic solution (instead of acidic), start by completing the same steps 1−3. Recall that during Step 4, you combined the half reactions:
Step 4:
Combine the half reactions in basic solution.
Then, add 16 OH− to both sides to neutralize the H+:
Combine the H+ and OH− into water:
Cancel out any species (here, e− and H2O) that appear on both sides to find the balanced redox reaction in a basic solution:
Step 5:
Finally, confirm that mass and charge are balanced. Here, there is a −12 net charge on each side of the reaction equation, and the atoms are stoichiometrically balanced.
Redox reactions can occur as variations of the major types of reactions above: combination, decomposition, and displacement.
| Example: | Equation | N2 (g) + 3 H2 (g) → 2 NH3 (g) | ||
| Oxidation #s | 0 | 0 | −3 +1 | |
| Example: | Equation | 2 H2O (l) → H2 (g) + O2 (g) | ||
| Oxidation #s | +1 −2 | 0 | 0 | |
| Example: | Equation | 2 Na (s) + 2 H2 O (l) → 2 NaOH + H2 (g) | |||
| Oxidation #s | 0 | +1 −2 | +1 −2 +1 | 0 | |