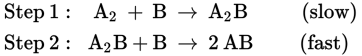
The mechanism of a chemical reaction is the actual series of steps through which it occurs. Knowing the accepted mechanism of a reaction often helps to explain the reaction’s rate, position of equilibrium, and thermodynamic characteristics. Consider the reaction below:
This equation seems to imply a mechanism in which two molecules of B collide with one molecule of A2 to form two molecules of AB. But suppose instead that the reaction actually takes place in two steps.
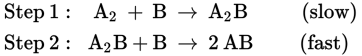
Note that these two steps add up to the overall (net) reaction. A2B, which does not appear in the overall reaction because it is neither a reactant nor a product, is called an intermediate. Reaction intermediates are often difficult to detect, but a proposed mechanism can be supported through experiments.
The slowest step in a proposed mechanism is called the rate-determining step, because the overall reaction cannot proceed faster than that step.