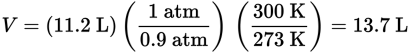Solutions to Review Problems
- D
Boyle’s law states that, when a gas is held at constant temperature, its pressure and volume are inversely proportional. This means that, as the pressure increases, the volume decreases, and vice versa. Of the answer choices, the only one that involves both pressure and volume—in addition to a controlled variation of one of the variables—is (D). When a balloon is placed in a bell jar, the volume of the balloon will increase as a vacuum is being drawn in the jar. Boyle’s law can be used to predict this behavior.
- A
In developing the kinetic molecular theory of gases, it was found that the properties of a gas sample, such as pressure, volume, and temperature, can be explained in terms of the motion of the individual gas molecules. Because kinetic is defined as “relating to motion,” this theory of gases was called the kinetic molecular theory.
- C
This question is an application of Charles’s law, which states that, at constant pressure, the volume and temperature of a gas will vary in direct proportion to each other. If a 50 L volume of gas is heated from standard temperature, which is 273 K, to two times standard temperature, 546 K, the volume will double as well. Therefore, the volume of the gas will increase from 50 L to 100 L. The answer can also be calculated using proportions.
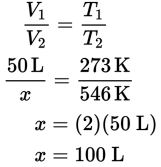
-
74.1 g/mol
The molecular weight of a gas is the number of grams that occupy 22.4 L at STP. At 630 torr and 600 K, the density of this gas is 2.5 g per 2 L. First, find the volume at STP:
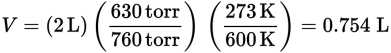
Next, find the density at STP:
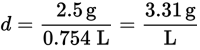
Finally, find the gram molecular weight of 22.4 L of gas at STP:
MM = d (V for 1 mol gas at STP) = (3.31 g/L)(22.4 L/mol) = 74.1 g/mol -
The conditions that define an ideal gas are low pressure and high temperature. Under these conditions, the gas molecules are assumed to have no intermolecular forces and to occupy no volume. Therefore, it is possible to predict their behavior.
- A
This question is an application of Avogadro’s principle, which states that, at a constant temperature and pressure, all gases will have the same number of moles in the same volume. This is true regardless of the identity of the gas. Thus, to answer the question, the number of particles in 0.25 mol of helium must be calculated; that value will represent the number of molecules of chlorine gas in the same volume. The number of helium atoms is (0.25 mol) (6.022 × 1,023 atoms/mol) = 1.51 × 1023 atoms. Thus, (D) is correct.
- D
The average speed of a gas is defined as the mathematical average of all the speeds of the gas particles in a sample. To answer this question, you must understand the Maxwell-Boltzmann distribution curve, which shows the distribution of speeds of all the gas particles in a sample at a given temperature. The distribution curve is a bell-shaped curve that flattens and shifts to the right as the temperature increases. The flattening of the curve means that gas particles within the sample are traveling at a greater range of speeds. As a result, a smaller proportion of the molecules will move at exactly the new average speed.
- A
A gas weighing 25 g/mol will have a density of 25 g per 22.4 L at STP. The density at 76 torr and 37°C is found by calculating the change in volume of a mole of gas under these conditions:
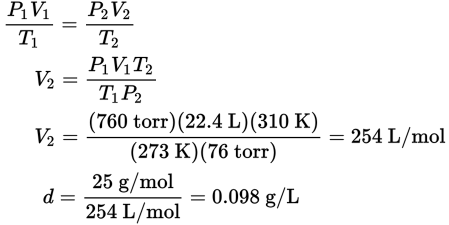
-
8.88 g
First, let’s find out how many moles of HCl would occupy 3 L at the pressure and temperature given.
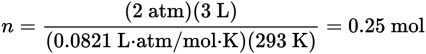
Because 2 mol of HCl are produced from each mol of Cl2, 0.25 mol HCl would be produced from 0.125 mol of Cl2. The molecular weight of Cl2 is 71 g/mol, so the answer is (71 g Cl2/mol) (0.125 mol) = 8.88 g Cl2.
- C
Discussed in the section on Dalton’s law of partial pressures in this chapter.
-
N2: 38 torr, O2: 152 torr, He: 152 torr, Cl2: 38 torr
According to Dalton’s law of partial pressures, the sum of the partial pressures of the gases in a mixture is equal to the total pressure of the mixture. Therefore, the partial pressures of nitrogen, oxygen, helium, and chlorine will add up to 380 torr. The partial pressure of a gas is calculated as follows:
Pp = XPT
where X is the mole fraction of the gas. Thus, the mole fractions of each of the gases must be determined.
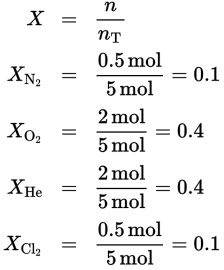
Now the partial pressures may be calculated.
PN2 = (380 torr)(0.1) = 38 torr
PO2 = (380 torr)(0.4) = 152 torr
PHe = (380 torr)(0.4) = 152 torr
PCl2 = (380 torr)(0.1) = 38 torr
-
20.2 g/mol
Convert the density given to the density at STP.
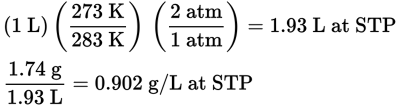
Now find the molar mass by multiplying by 22.4.
MM = (0.902 g/L)(22.4 L/mol) = 20.2 g/mol
- D
Kinetic energy is proportional to temperature, not number of moles. In an isolated system, increasing the number of moles of a gas while maintaining constant temperature and pressure will cause a decrease in temperature and therefore kinetic energy, indicating an inverse relationship.
-
0.4 atm
This question is different from the previous ones in that the new volume is given and you are looking for the final pressure. Rearranging the equation
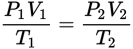
gives

-
13.7 L
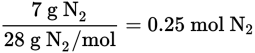
From the balanced reaction equation, 2 mol of NO2 are produced from each mol of N2, so 0.50 mol of NO2 will be produced from 0.25 mol of N2. Therefore, the volume at STP will be (0.5 mol NO2) (22.4 L/mol at STP) = 11.2 L NO2.
Now find the volume under the conditions given.