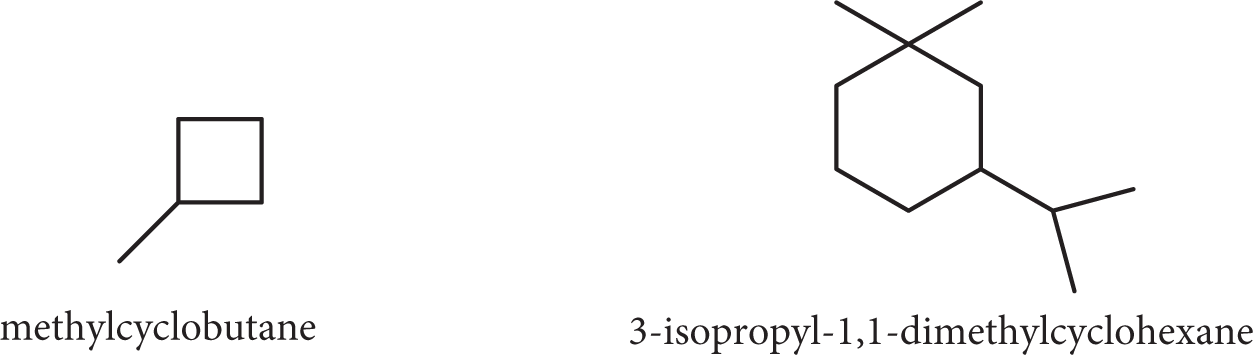Alkanes are the simplest organic molecules, consisting only of carbon and hydrogen atoms held together by single bonds.
The names of the four simplest alkanes are:
| CH4 | CH3CH3 | CH3CH2CH3 | CH3CH2CH2CH3 |
| methane | ethane | propane | butane |
The names of the longer-chain alkanes consist of prefixes derived from the Greek root for the number of carbon atoms with the ending -ane.
| C5H12 = pentane | C9H20 = nonane |
| C6H14 = hexane | C10H22 = decane |
| C7H16 = heptane | C11H24 = undecane |
| C8H18 = octane | C12H26 = dodecane |
These prefixes are applicable to more complex organic molecules, too, and should be memorized.
The International Union of Pure and Applied Chemistry (IUPAC) proposed a set of simple rules for naming complex molecules. This basic system can be used to name all classes of organic compounds. Throughout these notes, the IUPAC names will be listed as the primary name, and common names will appear in parentheses.
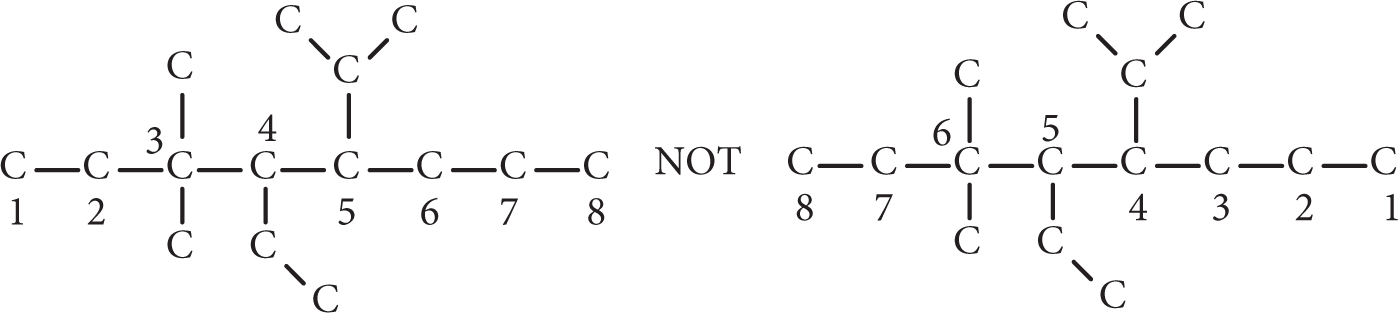
The longest continuous carbon chain within the compound is taken as the backbone. If two or more chains are of equal length, the most highly substituted chain (the one with the greatest number of other groups attached) takes precedence. The longest chain may not be as obvious from the structural formula as when it is drawn. For example, the backbone shown below is an octane (it contains eight carbon atoms).

Number the chain from one end in such a way that the lowest set of numbers is obtained for the substituents (which in alkanes are carbon groups not part of the main carbon chain).
Substituents are named according to their appropriate prefix with the ending -yl. For example:
| CH3– | CH3CH2– | CH3CH2CH2– |
| methyl | ethyl | n-propyl |
The prefix n- in the above example indicates an unbranched (“normal”) compound. There are special names for some common branched alkanes (see Figure 36.3), and these are usually used in the naming of substituents.

If two or more equivalent groups are present, the prefixes di-, tri-, tetra-, etc. are used.
Each substituent is assigned a number to identify its point of attachment to the principal chain. If the prefixes di-, tri-, tetra-, etc., are used, a number is still necessary for each individual group.
List the substituents in alphabetical order with their corresponding numbers. Prefixes such as di-, tri-, etc., as well as the hyphenated prefixes (tert- [or t-], sec-, n-), are ignored in alphabetizing. In contrast, cyclo-, iso-, and neo- are considered part of the group name and are alphabetized. Commas should be placed between numbers, and hyphens should be placed between numbers and words. For example:

You may also need to indicate the isomer you are describing—e.g., cis or trans, R or S, etc. For more information, see Chapter 37, Isomers.
Alkanes can also form rings rather than straight chains. These are named according to the number of carbon atoms in the ring with the prefix cyclo-.

Substituted cycloalkanes are named as derivatives of the parent cycloalkane. The substituents are named, and the carbon atoms are numbered around the ring starting from the point of greatest substitution. Again, the goal is to provide the lowest series of numbers as in rule number 2 above.