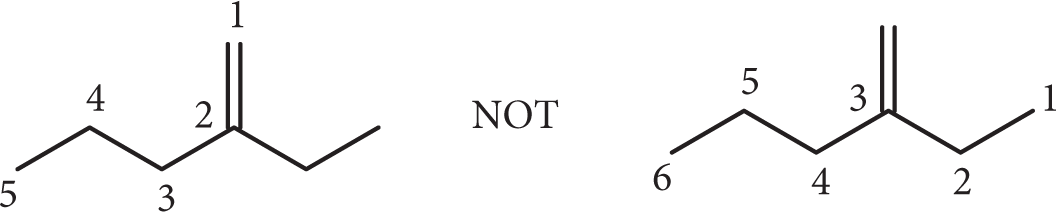
Organic molecules more complicated than simple alkanes can also be named using the 5-step process but with a few additional considerations.
Alkenes (or olefins) are compounds containing carbon-carbon double bonds. The nomenclature rules are essentially the same as for alkanes except that the ending -ene is used rather than -ane. Note the exceptions of the common names ethylene and propylene , which are used preferentially over the IUPAC names ethene and propene.
When identifying the carbon backbone, select the longest chain that contains the double bond (or the greatest number of double bonds if more than one is present).
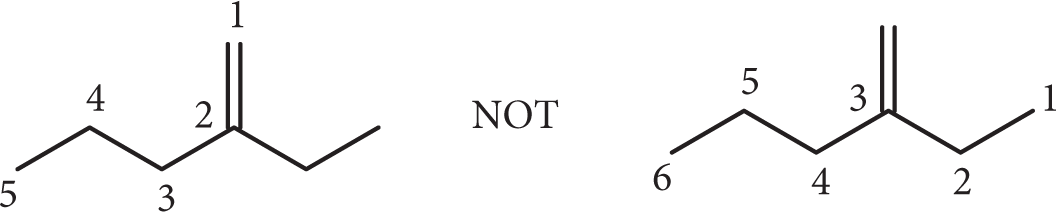
Number the backbone so the double bond receives the lowest number possible. Remember that multiple double bonds must be named using the prefixes di-, tri-, etc. and that each must receive a number. Also, you may need to name the configurational isomer (cis/trans, Z/E). This topic will be discussed further in Chapter 37, Isomers.
Substituents are named as they are for alkanes, and their positions are specified by the number of the backbone carbon atom to which they are attached.
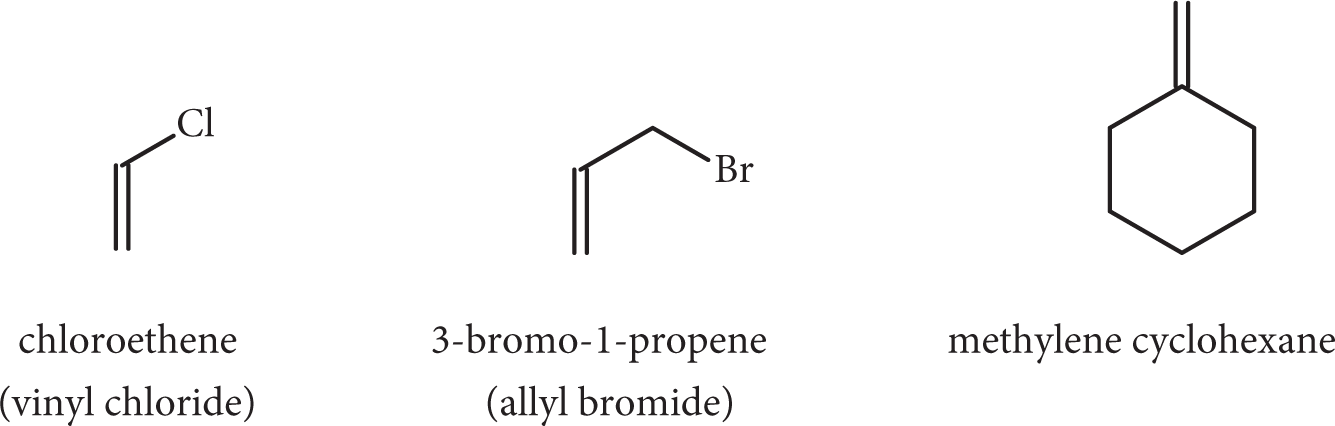
Frequently, an alkene group must be named as a substituent. In these cases, the systematic names may be used, but common names are more popular. Vinyl- derivatives are monosubstituted ethylenes (ethenyl-), and allyl- derivatives are propylenes substituted at the C–3 position (2-propenyl-). Methylene refers to the –CH2 group.
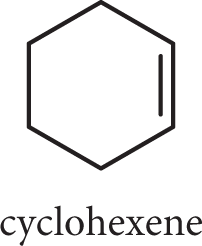
Cycloalkenes are named like cycloalkanes but with the suffix -ene rather than -ane. If the molecule has only one double bond and no other substituents, a number is not necessary.
Alkynes are compounds that possess carbon-carbon triple bonds. The suffix -yne replaces -ane in the parent alkane. The position of the triple bond is indicated by a number when necessary. The common name for ethyne is acetylene, and this name is used almost exclusively.
