Review Problems
-
Under the following conditions,

1-bromo-4-methylpentane will most probably react via
- SN1.
- SN2.
- both SN1 and SN2.
- neither SN1 nor SN2.
-
The following molecule can be classified as having

- 4 primary, 2 secondary, 4 tertiary, and 3 quaternary carbon atoms.
- 3 methyl groups, 2 ethyl groups, and 4 secondary carbon atoms.
- 4 primary, 6 secondary, 2 tertiary, and 1 quaternary carbon atoms.
- 3 primary, 3 secondary, 4 tertiary, and 3 quaternary carbon atoms.
-
The following reactions are part of a free-radical halogenation sequence:
ΔH (kcal/mol) (A) Cl2→ 2 Cl• +58 (B) Cl• + CH4→ •CH3 + HCl +1 (C) •CH3 + Cl2→ CH3Cl + Cl• −26 (D) •CH3 + Cl• → CH3Cl −84 Identify the initiation, propagation, and termination steps.
-
SN1 reactions show first-order kinetics because
- the rate-limiting step is the first step to occur in the reaction.
- the rate-limiting step involves only one molecule.
- there is only one rate-limiting step.
- the reaction involves only one molecule.
-
The following reaction sequence is typical of SN1 reactions. Which is the rate-limiting step(s)?
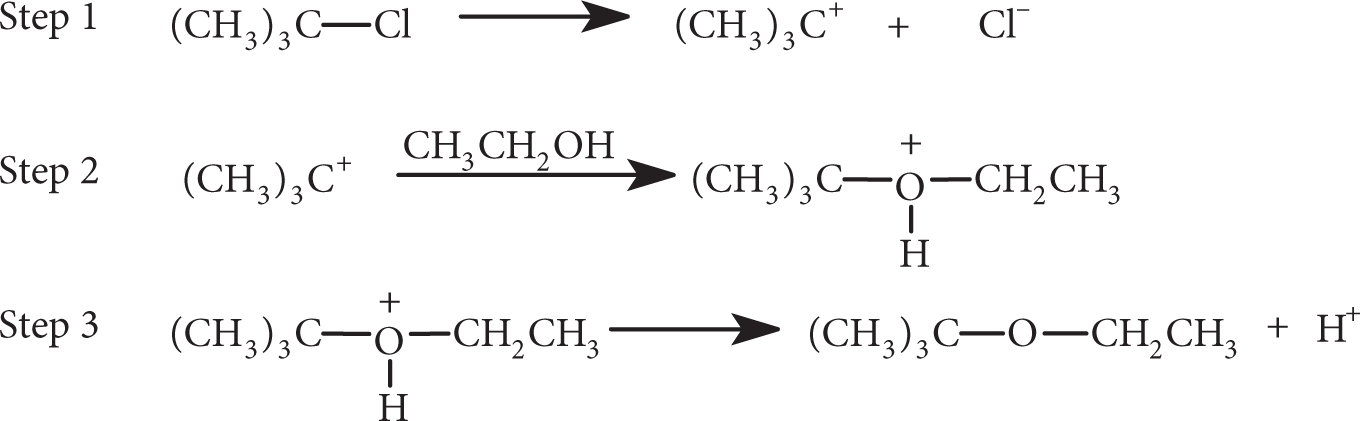
- Step 1
- Step 2
- Step 3
- Steps 1 and 2
-
Which of the following would be the best solvent for an SN2 reaction?
- H2O
- CH3CH2OH
- CH3SOCH3
- CH3CH2CH2CH2CH2CH3
-
What choices for X and Y would most favor the following reaction?

- X = I−, Y = Cl−
- X = EtO−, Y = tosylate (CH3C6H4SO2)
- X = tosylate, Y = CN−
- X = OH−, Y = H2O
-
What would be the major product of the following reaction?
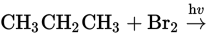
- CH3CH2CH2Br
- CH3CH2CH2CH2CH2CH3
- H3CCHBrCH3
- CH3CH2Br
-
Treatment of (S)-2-bromobutane with sodium hydroxide results in the production of a compound with an (R) configuration. The reaction has most likely taken place through
- an SN1 mechanism.
- an SN2 mechanism.
- both an SN1 and SN2 reaction.
- a reaction that cannot be determined.
-
What is the correct decreasing order of the boiling points of the following compounds?
- n-hexane
- 2-methylpentane
- 2,2-dimethylbutane
- n-heptane
- I > IV > II > III
- IV > III > II > I
- IV > I > II > III
- I > II > III > IV
-
The reaction of isobutane with an unknown halogen is catalyzed by light. The two major products obtained are:
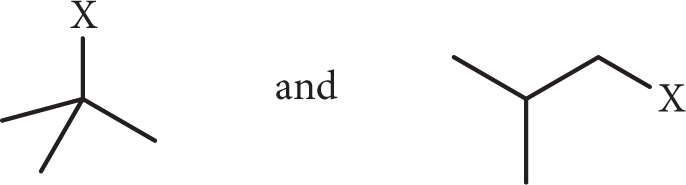
What is the unknown halogen?
- Cl2
- Br2
- I2
- F2
-
Place the following species in order of increasing stability.
-
- CH3CH2CH2•
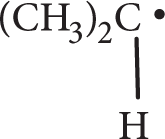
- CH3•
- (CH3)3C•
-

- R3C+
- H3C+

-