Alkynes undergo several types of reactions, most of which are closely related to the reactions of alkenes:
Alkynes, just like alkenes, can be hydrogenated with a metal catalyst to produce alkanes in a reaction that goes to completion:
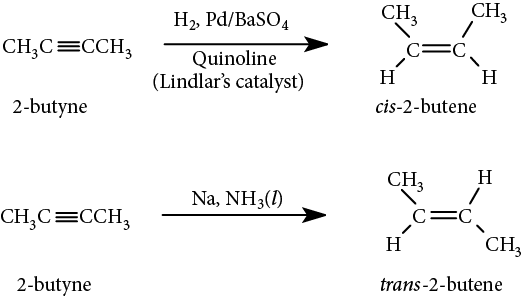
A more useful reaction stops the reduction after addition of just one equivalent of H2, producing alkenes. This partial hydrogenation can take place in two different ways. The first uses Lindlar’s catalyst, which is palladium on barium sulfate (BaSO4) with quinoline, a poison that stops the reaction at the alkene stage. Because the reaction occurs on a metal surface, the product alkene is the cis isomer. The other method uses sodium in liquid ammonia below −33°C (the boiling point of ammonia), and produces the trans isomer of the alkene via a free radical mechanism:

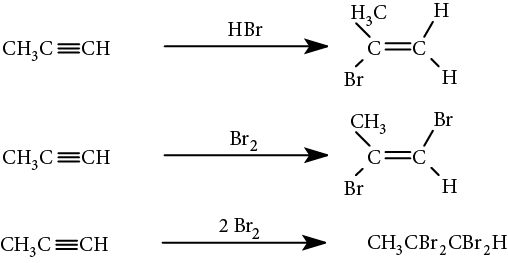
Electrophilic addition to alkynes occurs in the same manner as it does to alkenes, with the reaction following Markovnikov’s rule. The addition can generally be stopped at the intermediate alkene stage, or carried further:
Radicals add to triple bonds as they do to double bonds—with anti-Markovnikov placement of the halogen. The reaction product is usually the trans isomer, because the intermediate vinyl radical can isomerize to its more stable form as shown:

Addition of boron to triple bonds occurs by the same method as addition of boron to double bonds. Addition is syn, and the boron atom adds first. The boron atom can be replaced with a proton from acetic acid, to produce a cis alkene:
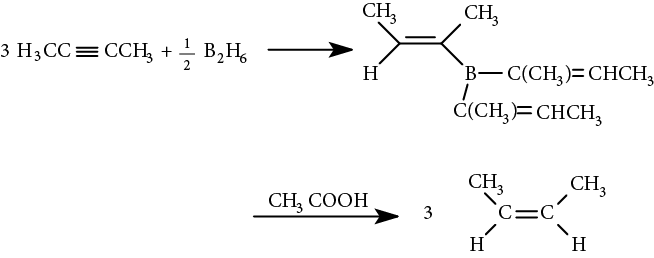
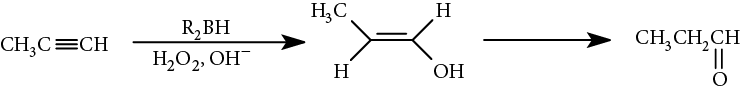
With terminal alkynes, a disubstituted borane is used to prevent further boration of the vinylic intermediate to an alkane. The vinylic borane intermediate can be oxidatively cleaved with hydrogen peroxide (H2O2), creating an intermediate vinyl alcohol, which rearranges to the more stable carbonyl compound via keto-enol tautomerism (discussed in Chapter 43, Aldehydes and Ketones).
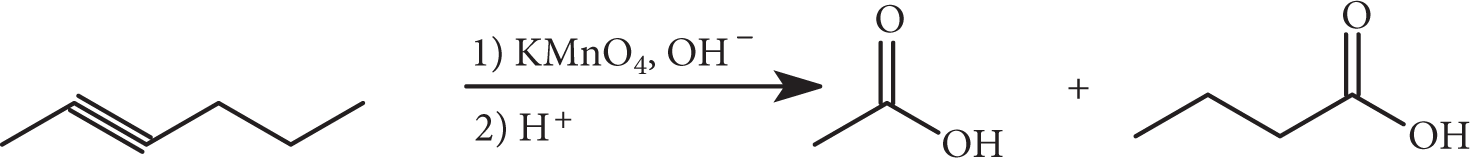
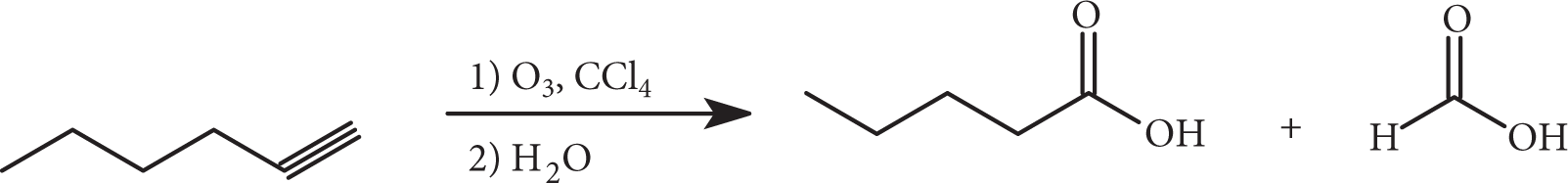
Alkynes can be oxidatively cleaved with either basic potassium permanganate (followed by acidification) or ozone. In both instances shown, carboxylic acids are produced, but treatment of a terminal alkyne with basic permanganate will produce carbon dioxide.