Ethers react with the oxygen in air to form highly explosive compounds called peroxides (general formula ROOR). For this reason, ethers are typically stored at low temperature and away from light.
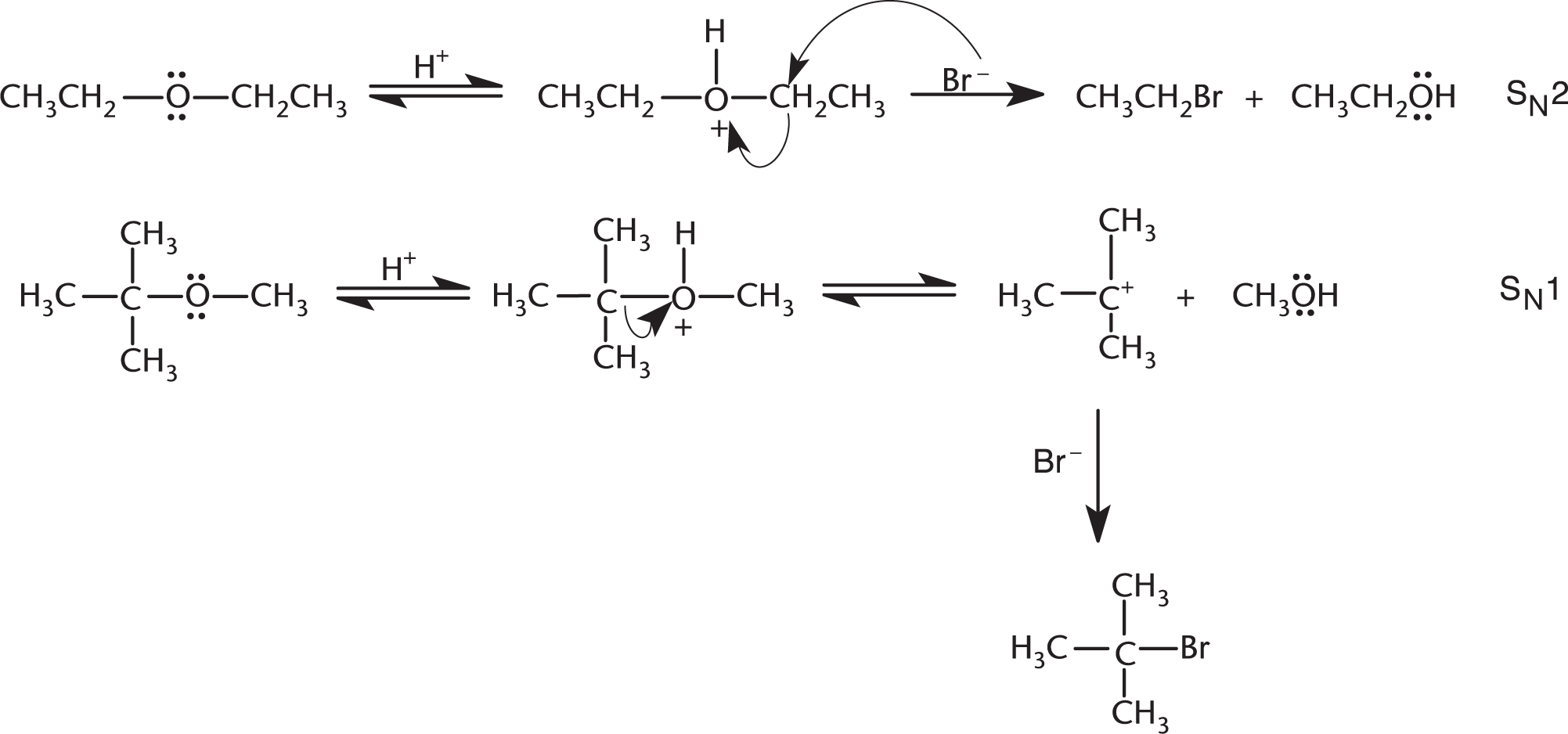
Cleavage of straight-chain ethers will take place only under vigorous conditions: usually at high temperatures in the presence of HBr or HI. Cleavage is initiated by protonation of the ether oxygen. The reaction then proceeds by an SN1 or SN2 mechanism, depending on the conditions and the structure of the ether (note the difference in substitution of the substrates in Figure 42.24). Although not shown below, the alcohol products usually react with a second molecule of hydrogen halide to produce a second alkyl halide.

The same basic principles and reaction mechanisms of substitution reactions apply to epoxides. Since epoxides are highly strained cyclic ethers, they are susceptible to SN2 reactions. Unlike straight-chain ethers, these reactions can be catalyzed by acids or bases. In symmetrical epoxides, either carbon can be attacked by a nucleophile; however, in asymmetrical epoxides, the most substituted carbon is attacked by the nucleophile in the presence of acid, and the least substituted carbon is attacked in the presence of base:

Reactions of epoxides provide additional insight into SN1 and SN2 reaction mechanisms. Base-catalyzed cleavage of epoxides has the most SN2 character, so it occurs at the least hindered (least substituted) carbon. The basic environment provides the strong nucleophile required for SN2 reactions.
In contrast, acid-catalyzed cleavage is thought to have some SN1 character as well as some SN2 character. The epoxide oxygen can be protonated, making it a better leaving group. This gives the carbons partial positive charges. Since substitution stabilizes this charge (3° carbons provide the most stable carbocations), the more substituted C becomes a good target for nucleophilic attack.