Alcohols can be prepared from a variety of different types of compounds. Methanol, also called wood alcohol, is obtained from the destructive distillation of wood. It is toxic and can cause blindness if ingested. Ethanol, or grain alcohol, is produced from the fermentation of sugars and can be metabolized by the body; however, in large enough quantities, it too is toxic.
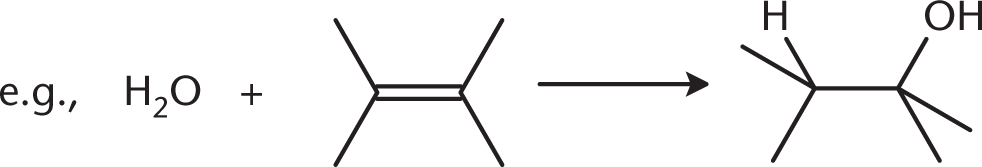
Alcohols can be prepared via several reactions that involve addition of water to double bonds (discussed in Chapter 41).

Alcohols can also be prepared from the addition of organometallic compounds to carbonyl groups (discussed in Chapter 46).

Both SN1 and SN2 reactions can be used to produce alcohols under the appropriate reaction conditions (discussed in Chapter 42). For example:
Alcohols can be prepared from the reduction of aldehydes, ketones, carboxylic acids, or esters. Lithium aluminum hydride (LiAlH4, or LAH) and sodium borohydride (NaBH4) are the two most frequently used reducing reagents. LAH is stronger and less specific, whereas NaBH4 is milder and more selective. For example, LAH will reduce carboxylic acids and esters to alcohols, while NaBH4 will not. Both LAH and NaBH4, however, will reduce aldehydes and ketones to alcohols.

Phenols may be synthesized from arylsulfonic acids with heat and NaOH. However, this reaction is useful only for phenol or its alkylated derivatives, as most functional groups are destroyed by the harsh reaction conditions.
A more versatile method of synthesizing phenols proceeds using hydrolysis of diazonium salts.
