Carboxylic acids are polar and form hydrogen bonds between the –OH group of one molecule and the lone pairs of electrons on the oxygen of another molecule. As a result, carboxylic acids can form dimers: pairs of molecules connected by hydrogen bonds. The boiling points of carboxylic acids are therefore even higher than those of the corresponding alcohols. The boiling points follow the usual trend of increasing with molecular weight.
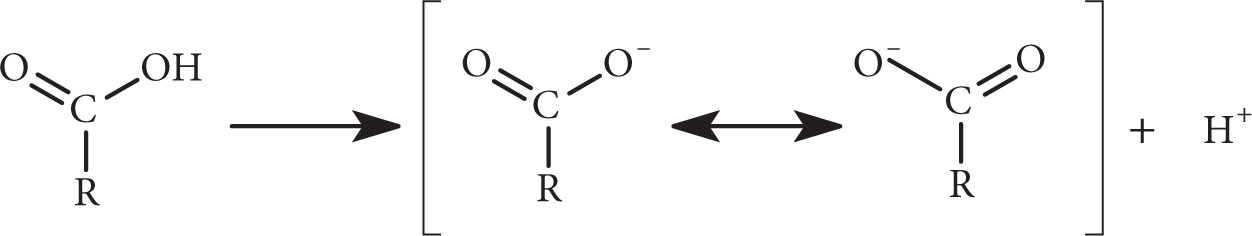
The acidity of carboxylic acids is due to the resonance stabilization of the carboxylate anion (the conjugate base). When the hydroxyl proton dissociates from the acid, the negative charge left on the carboxylate group is delocalized and shared by both oxygen atoms.

Substituents on carbon atoms adjacent to a carboxyl group can influence acidity. Electron-withdrawing groups such as –Cl or –NO2 further delocalize the negative charge and increase acidity. Electron-donating groups such as –NH2 or –OCH3 destabilize the negative charge, making the compound less acidic.
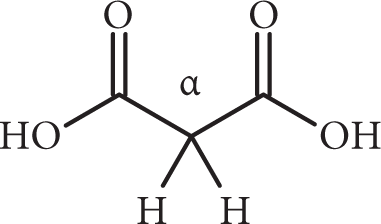
In dicarboxylic acids, one –COOH group (which is electron-withdrawing) influences the other, making the first carboxyl group more acidic than the analogous monocarboxylic acid. The second carboxyl group is then influenced by the carboxylate anion. Ionization of the second group will create a doubly charged species, in which the two negative charges repel each other. Since this is unfavorable, the second proton is less acidic than that of a monocarboxylic acid.
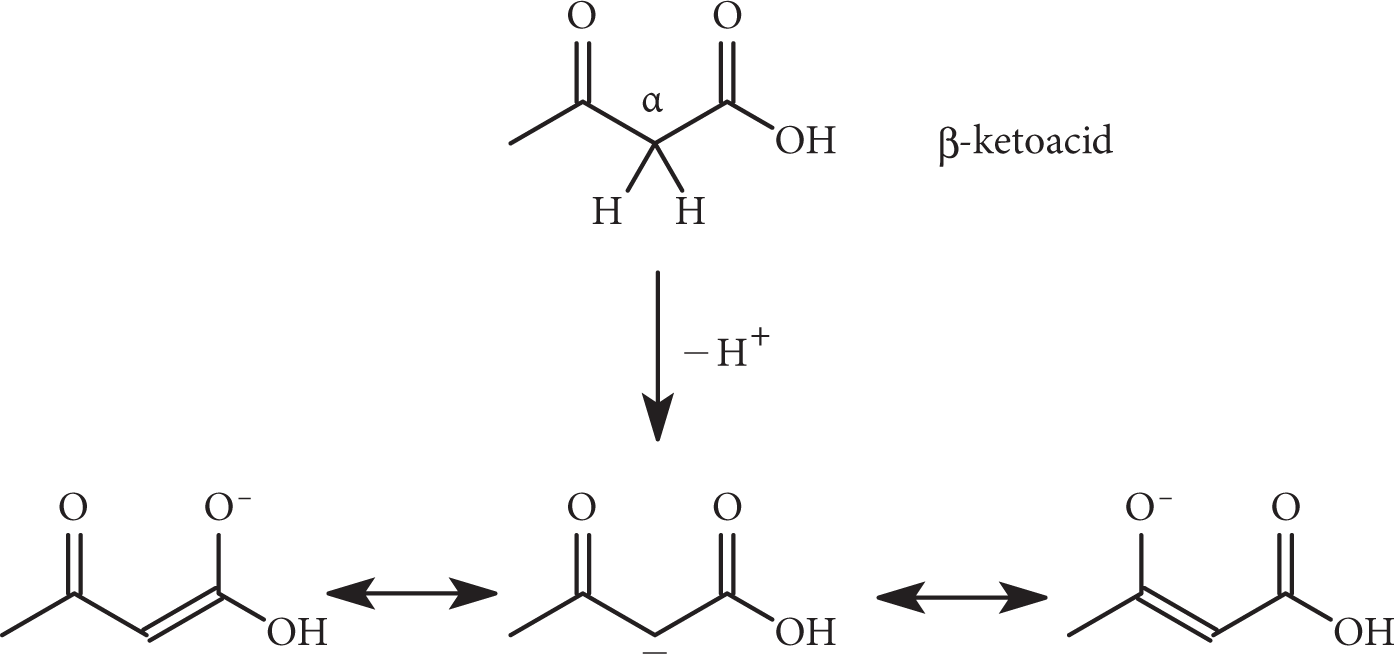
β-dicarboxylic acids are notable for the high acidity of the α-hydrogens located between the two carboxyl groups (pKa ~ 10). Loss of this acidic hydrogen atom produces a carbanion that is stabilized by the electron-withdrawing effect of the two carboxyl groups.
The same effect is also seen in β-ketoacids, RC═OCH2–COOH. The resonance structures of the representative β-ketoacid below demonstrate the additional stability due to delocalization of the electrons from the carbonyl groups: